|
Free Radical Damage
Free radical damage to DNA can occur as a result of exposure to ionizing radiation or to radiomimetic compounds. Damage to DNA as a result of free radical attack is called indirect DNA damage because the radicals formed can diffuse throughout the body and affect other organs. Malignant melanoma can be caused by indirect DNA damage because it is found in parts of the body not exposed to sunlight. DNA is vulnerable to radical attack because of the very labile hydrogens that can be abstracted and the prevalence of double bonds in the DNA bases that free radicals can easily add to. Damage via radiation exposure Radiolysis of intracellular water by ionizing radiation creates peroxides, which are relatively stable precursors to hydroxyl radicals. 60%–70% of cellular DNA damage is caused by hydroxyl radicals, yet hydroxyl radicals are so reactive that they can only diffuse one or two molecular diameters before reacting with cellular components. Thus, hydroxyl radicals must be forme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionizing Radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum. Gamma rays, X-rays, and the higher energy ultraviolet part of the electromagnetic spectrum are ionizing radiation, whereas the lower energy ultraviolet, visible light, nearly all types of laser light, infrared, microwaves, and radio waves are non-ionizing radiation. The boundary between ionizing and non-ionizing radiation in the ultraviolet area is not sharply defined, as different molecules and atoms ionize at different energies. The energy of ionizing radiation starts between 10 electronvolts (eV) and 33 eV. Typical ionizing subatomic particles include alpha particles, beta particles, and neutrons. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mutagenic
In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in animals, such mutagens can therefore be carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes. The process of DNA becoming modified is called mutagenesis. Not all mutations are caused by mutagens: so-called "spontaneous mutations" occur due to spontaneous hydrolysis, errors in DNA replication, repair and recombination. Discovery The first mutagens to be identified were carcinogens, substances that were shown to be linked to cancer. Tumors were described more than 2,000 years before the discovery of chromosomes and DNA; in 500 B.C., the Greek physician Hippocrates named tumors resembling a crab ''karkinos'' (from which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
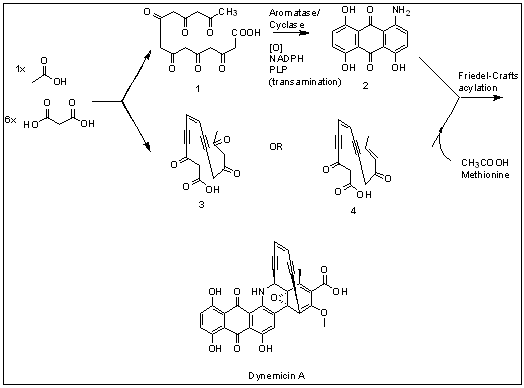
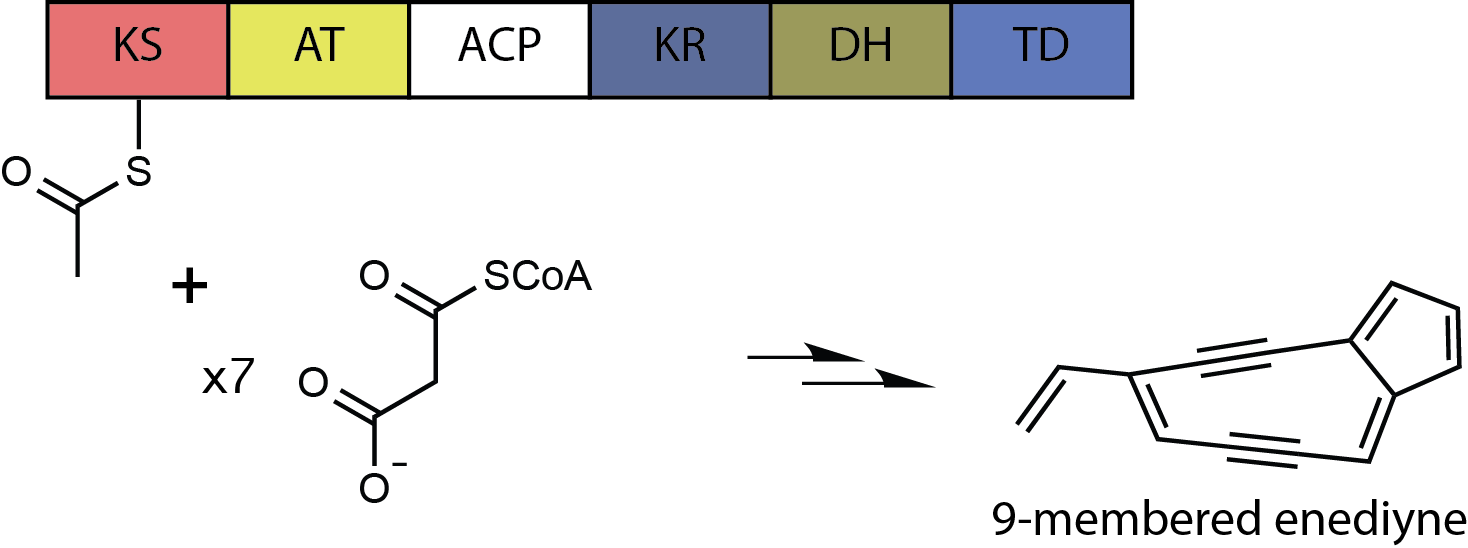
Dynemicin A
Dynemicin A is an anti-cancer enediyne drug. It displays properties which illustrate promise for cancer treatments, but still requires further research. History and background Dynemicin A was first isolated from the soil in the Gujarat State of India. It was discovered to be the natural product of the indigenous bacteria '' Micromonospora chersina''. The natural product displays a bright purple color due to the anthraquinone chromophore structure within it. Initially, this compound was isolated for its aesthetic properties as a dye until further research demonstrated its anti-cancer properties. Shortly after the compound's discovery, the Bristol-Myers Pharmaceutical Company elucidated the structure in Japan using X-ray diffraction studies of triacetyldynemicin A; a closely related compound. Synthesis The first reported chemical synthesis of dynemicin was accomplished by Myers and coworkers. Biosynthesis Dynemicin A is an antitumor natural product isolated from ''Micro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calicheamicin
The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium ''Micromonospora echinospora'', with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs. It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML). A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin (marketed as Besponsa) an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Calicheamicin γ1 and the related enediyne esperamicin are the two of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Different Mechanisms Of DNA Cleavage By P-benzyne
Different may refer to: Music * ''Different'' (Thomas Anders album), 1989 * ''Different'' (Kate Ryan album), 2002 * "Different" (Band-Maid song), 2020 * "Different" (Robbie Williams song), 2012 * "Different", a song by Acceptance from the 2005 album '' Phantoms'' * "Different", a song by Burna Boy from the 2019 album ''African Giant'' * "Different", a song by Cass Elliot from the soundtrack of the 1970 film '' Pufnstuf'' * "Different", a song by Dreamscape from the 2007 album ''5th Season'' * "Different", a song by Egypt Central from the 2005 album Egypt Central * "Different", a song by Future and Juice Wrld from the 2018 mixtape ''Wrld on Drugs'' * "Different", a 2006 song by Jamie Shaw * "Different", a 2017 song by Micah Tyler * "Different", a song by No Malice from the 2013 album ''Hear Ye Him'' * "Different", a song by Pendulum from the 2008 album ''In Silico'' * "Different", a song by Winner from the 2014 album ''2014 S/S'' * "Different", a song by Ximena Sariñana from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-benzyne Generation
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydroarenes in this case), although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes. Bonding in arynes The alkyne representation of benzyne is the most widely encountered. Arynes are usually described as having a strained triple bond. Geometric constraints on the triple bond in benzyne result in diminished overlap of in-plane p-orbitals, and thus weaker triple bond. The vibrational frequency of the triple bond in benzyne was assigned by Radziszewski to be 1846 cm−1, indicating a weaker triple bond than in unstrained alkyne with vibrational frequency of approximately 2150 cm−1. Nevertheless, benzyne is more like a strained alkyne than a diradical, as seen from the large singlet–triplet gap and alkyne-like reactivity. The LUMO of aryne lies much lower than the LUMO of un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryne
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydroarenes in this case), although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes. Bonding in arynes The alkyne representation of benzyne is the most widely encountered. Arynes are usually described as having a strained triple bond. Geometric constraints on the triple bond in benzyne result in diminished overlap of in-plane p-orbitals, and thus weaker triple bond. The vibrational frequency of the triple bond in benzyne was assigned by Radziszewski to be 1846 cm−1, indicating a weaker triple bond than in unstrained alkyne with vibrational frequency of approximately 2150 cm−1. Nevertheless, benzyne is more like a strained alkyne than a diradical, as seen from the large singlet–triplet gap and alkyne-like reactivity. The LUMO of aryne lies much lower than the LUMO of un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bergman Cyclization
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen donor (''Scheme 1''). It is the most famous and well-studied member of the general class of cycloaromatization reactions. It is named for Japanese-American chemist Satoru Masamune (b. 1928) and American chemist Robert G. Bergman (b. 1942). The reaction product is a derivative of benzene. The reaction proceeds by a thermal reaction or pyrolysis (above 200 °C) forming a short-lived and very reactive para-benzyne biradical species. It will react with any hydrogen donor such as 1,4-cyclohexadiene which converts to benzene. When quenched by tetrachloromethane the reaction product is a 1,4-dichlorobenzene and with methanol the reaction product is benzyl alcohol. When the enyne moiety is incorporated into a 10-membered hydrocarbon rin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enediyne
In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond. Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne Chemotherapy, anticancer antibiotics). They are efficient at inducing apoptosis in Cell biology, cells, but cannot differentiate Cancer, cancerous cells from healthy cells. Consequently, research is being conducted to increase the specificity of enediyne toxicity. Structure and reactivity A nine- or ten-membered ring containing a double bond between two triple bonds is termed the warhead of the enediyne. In this state, the warhead is inactive. Enediynes are triggered into a chemically active state via Bergman cyclization, Bergman or Myers-Saito cyclization. The triggering mechanism can be attributed to an intramolecular nucleophilic attack initiated by one of the variable regions of the molecule. Triggering can also occur via attack by an external nucleophile. Bergman cyclization re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |