|
Fluxionality
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening (beyond that dictated by the Heisenberg uncertainty principle) due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods. Spectroscopic studies Many organometallic compounds exhibit fluxionality. Fluxionality is however pervasive. NMR spectroscopy Temperature dependent changes in the NMR spectra result from dynamics associated with the fluxional molecules when those dynamics proceed at rates compara ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transfer
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons. Electrochemical processes are ET reaction. ET reactions are relevant to photosynthesis and respiration. ET reactions commonly involve transition metal complexes, In organic chemistry ET is a step in some commercial polymerization reactions. It is foundational to photoredox catalysis. Classes of electron transfer Inner-sphere electron transfer In inner-sphere ET, the two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanium
In chemistry, methanium is a complex positive ion with formula []+, namely a molecule with one carbon atom covalent bond, bonded to three hydrogen atoms and one hydrogen molecule, bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion. It is highly unstable and highly reactive even upon having a complete octet, thus granting its superacidic properties. Methanium can be produced in the laboratory as a rarefied gas or as a dilute species in superacids. It was prepared for the first time in 1950 and published in 1952 by Victor Talrose and his assistant Anna Konstantinovna Lyubimova. It occurs as an intermediate species in chemical reactions. The methanium ion is named after methane (), by analogy with the derivation of ammonium ion () from ammonia (). Structure Fluxional methanium can be visualised as a carbenium ion with a molecule of hydrogen interacting with the empty orbital in a 3-center-2-electron bond. The bondi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
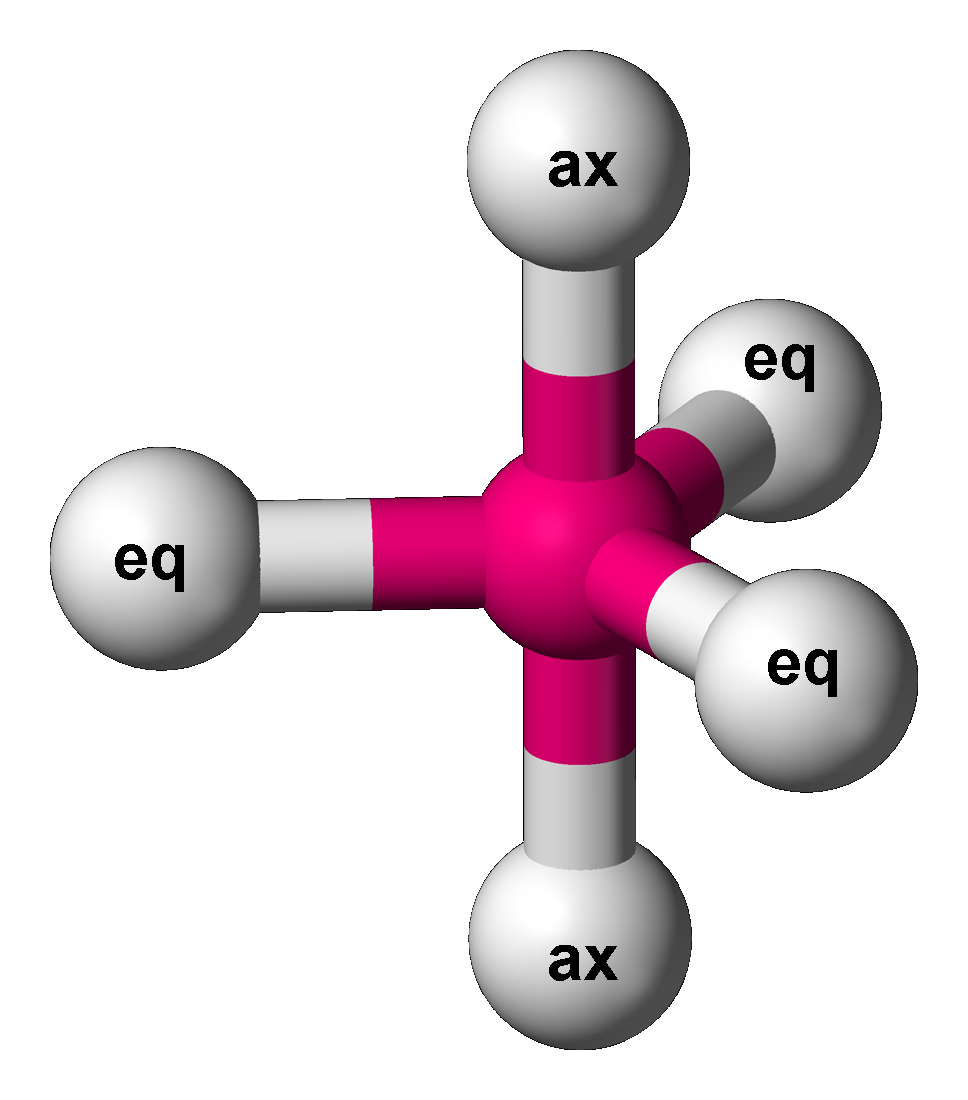
Sulfur Tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the formal +4 oxidation state. Of sulfur's total of six valence electrons, two form a lone pair. The structure of SF4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula . Under standard conditions Fe( CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis. Properties Iron pentacarbonyl is a homoleptic metal carbonyl, where carbon monoxide is the only ligand complexed with a metal. Other examples include octahedral Cr(CO)6 and tetrahedral Ni(CO)4. Most metal carbonyls have 18 valence electrons, and Fe(CO)5 fits this pattern with 8 valence electrons on Fe and five pairs of electrons provided by the CO ligands. Reflecting its symmetrical structure and charge neutrality, Fe(CO)5 is volatile; it is one of the most frequently encountered liquid metal complexes. Fe(CO)5 adopts a trigonal bipyramidal structure with the Fe atom surrounded by five CO ligands: three in equatorial positions and two axially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berry Mechanism
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see Figure at right) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.RS Berry, 1960, "Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds," ''J. Chem. Phys.'' 32:933-938, DOI 10.1063/1.1730820; seo accessed 28 May 2014M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Ed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chemistry (IUPAC) defines a pseudorotation as a " ereoisomerization resulting in a structure that ''appears'' to have been produced by rotation of the entire initial molecule", the result of which is a "product" that is "superposable on the initial one, unless different positions are distinguished by substitution, including isotopic substitution." Well-known examples are the intramolecular isomerization of trigonal bipyramidal compounds by the Berry pseudorotation mechanism, and the out-of-plane motions of carbon atoms exhibited by cyclopentane, leading to the interconversions it experiences between its many possible conformers (envelope, twist). Note, no angular momentum is generated by this motion. In these and related examples, a small ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
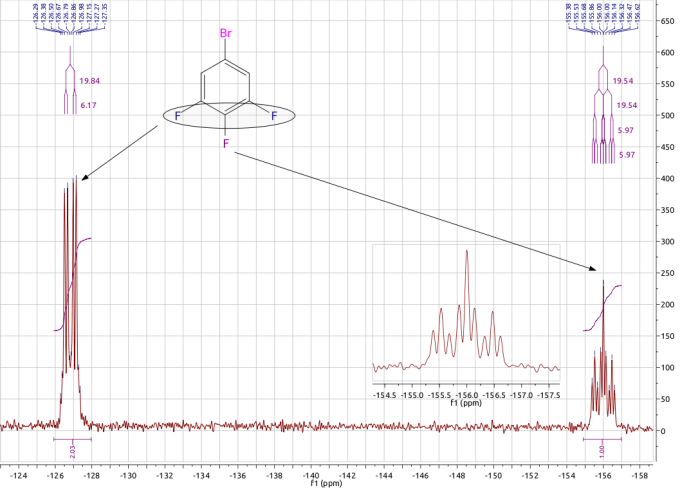
Fluorine-19 Nuclear Magnetic Resonance Spectroscopy
Fluorine-19 nuclear magnetic resonance spectroscopy (fluorine NMR or 19F NMR) is an analytical technique used to detect and identify fluorine-containing compounds. 19F is an important nucleus for NMR spectroscopy because of its receptivity and large chemical shift dispersion, which is greater than that for proton nuclear magnetic resonance spectroscopy. Operational details 19F has a nuclear spin (I) of and a high gyromagnetic ratio. Consequently, this isotope is highly responsive to NMR measurements. Furthermore, 19F comprises 100% of naturally occurring fluorine. The only other highly sensitive spin NMR-active nuclei that are monoisotopic (or nearly so) are 1H and 31P. Indeed, the 19F nucleus is the third most receptive NMR nucleus, after the 3H nucleus and 1H nucleus. The 19F NMR chemical shifts span a range of ''ca.'' 800 ppm. For ''organo''fluorine compounds the range is narrower, being ''ca.'' -50 to -70 ppm (for CF3 groups) to -200 to -220 ppm (for CH2F groups). Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine-19 Nuclear Magnetic Resonance Spectroscopy
Fluorine-19 nuclear magnetic resonance spectroscopy (fluorine NMR or 19F NMR) is an analytical technique used to detect and identify fluorine-containing compounds. 19F is an important nucleus for NMR spectroscopy because of its receptivity and large chemical shift dispersion, which is greater than that for proton nuclear magnetic resonance spectroscopy. Operational details 19F has a nuclear spin (I) of and a high gyromagnetic ratio. Consequently, this isotope is highly responsive to NMR measurements. Furthermore, 19F comprises 100% of naturally occurring fluorine. The only other highly sensitive spin NMR-active nuclei that are monoisotopic (or nearly so) are 1H and 31P. Indeed, the 19F nucleus is the third most receptive NMR nucleus, after the 3H nucleus and 1H nucleus. The 19F NMR chemical shifts span a range of ''ca.'' 800 ppm. For ''organo''fluorine compounds the range is narrower, being ''ca.'' -50 to -70 ppm (for CF3 groups) to -200 to -220 ppm (for CH2F groups). Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentafluoride
Phosphorus pentafluoride, P F5, is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Preparation Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride, which remains a favored method: :3 PCl5 + 5 AsF3 → 3 PF5 + 5 AsCl3 Structure Single-crystal X-ray studies indicate that the PF5 has trigonal bipyramidal geometry. Thus it has two distinct types of P−F bonds (axial and equatorial): the length of an axial P−F bond is distinct from the equatorial P−F bond in the solid phase, but not the liquid or gas phases due to Berry pseudo rotation. Fluorine-19 NMR spectroscopy, even at temperatures as low as −100 °C, fails to distinguish the axial from the equatorial fluorine environments. The apparent equivalency arises from the low barrier for pseudorotation via the Berry mechanism, by which the axial and equatorial fluorine atoms rapidly exchange pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H (hydrogen-1; i.e. having a proton for a nucleus). Simple NMR spectra are recorded in solution, and solvent protons must not be allowed to interfere. Deuterated (deuterium = 2H, often symbolized as D) solvents especially for use in NMR are preferred, e.g. deuterated water, D2O, deuterated acetone, (CD3)2CO, deuterated methanol, CD3OD, deuterated dimethyl sulfoxide, (CD3)2SO, and deuterated chloroform, CDCl3. However, a solvent without hydrogen, such as carbon tetrachloride, CCl4 or carbon disulfide, CS2, may also be used. Historically, deuterated solvents were supplied with a small amount (typically 0.1%) of tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |