|
Fluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perchlorate anion, , which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. As an anion in inorganic and organic chemistry The popularization of has led to decreased use of in the laboratory as a weakly coordinating anion. With organic compounds, especially amine derivatives, forms potentially explosive derivatives. Disadvantages to include its slight sensitivity to hydrolysis and decomposition via loss of a fluoride ligand, whereas does not suffer from these problems. Safety considerations, however, overshadow this inconvenience. With a formula weight of 86.8, BF is also conveni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroboric Acid
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an inorganic compound with the chemical formula +BF4−], where H+ represents the solvated proton. The solvent can be any suitably Lewis-basic entity. For instance, in water, it can be represented by (oxonium tetrafluoroborate), although more realistically, several water molecules solvate the proton: (H2O)''n''+BF4−]. The ethyl ether solvate is also commercially available: (Et2O)''n''+BF4−], where ''n'' is most likely 2. Unlike other strong acids like H2SO4 or HClO4, the pure unsolvated substance does not exist (see below). It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Balz–Schiemann Reaction
The Balz–Schiemann reaction (also called the Schiemann reaction) is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate. This reaction is a traditional route to fluorobenzene and some related derivatives, including 4-fluorobenzoic acid. : The reaction is conceptually similar to the Sandmeyer reaction, which converts diazonium salts to other aryl halides (ArCl, ArBr). However, while the Sandmeyer reaction involves a copper reagent/catalyst and radical intermediates, the thermal decomposition of the diazonium tetrafluoroborate proceeds without a promoter and is believed to generate highly unstable aryl cations (Ar+), which abstract F− from BF4− to give the fluoroarene (ArF), along with boron trifluoride as the byproduct. Innovations The traditional Balz–Schiemann reaction employs HBF4 and involves isolation of the diazonium salt. Both aspects can be profitably modified. Other counterion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
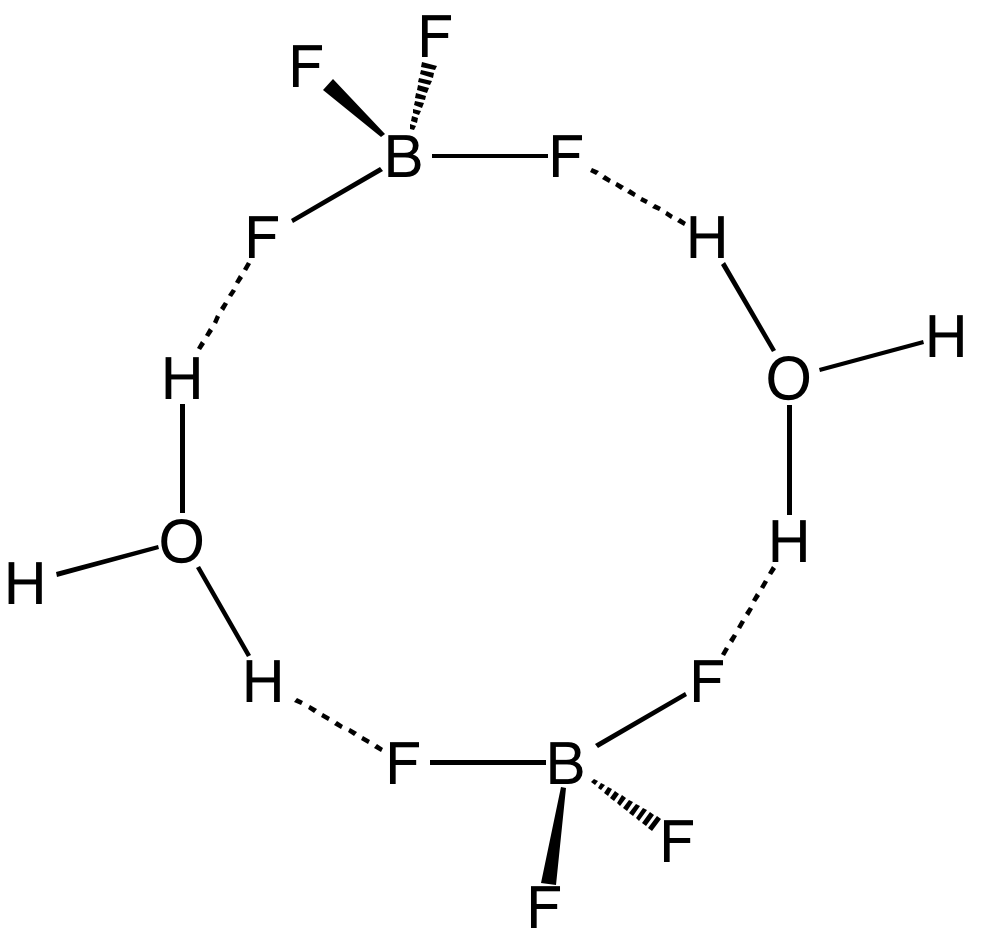
Boron Trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds. Structure and bonding The geometry of a molecule of BF3 is trigonal planar. Its D3h symmetry conforms with the prediction of VSEPR theory. The molecule has no dipole moment by virtue of its high symmetry. The molecule is isoelectronic with the carbonate anion, . BF3 is commonly referred to as " electron deficient," a description that is reinforced by its exothermic reactivity toward Lewis bases. In the boron trihalides, BX3, the length of the B–X bonds (1.30 Å) is shorter than would be expected for single bonds, and this shortness may indicate stronger B–X π-bonding in the fluoride. A facile explanation invokes the symmetry-allowed overlap of a p orbital on the boron atom with the in-phase combination of the three similarly oriented p orbitals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-coordinating Anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations C5H5)2ZrRsup>+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligands. N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroberyllate
Tetrafluoroberyllate or orthofluoroberyllate is an anion containing beryllium and fluorine. The fluoroanion has a tetrahedral shape, with the four fluorine atoms surrounding a central beryllium atom. It has the same size and outer electron structure as sulfate. Therefore, many compounds that contain sulfate have equivalents with tetrafluoroberyllate. Examples of these are the langbeinites, and Tutton's salts. Properties The Be–F bond length is between 145 and 153 pm. The beryllium has sp3 atomic hybridization, leading to a longer bond than in BeF2, where the Be is sp hybridized. In trifluoroberyllates, there are actually BeF4 tetrahedra arranged in a triangle, so that three fluorine atoms are shared on two tetrahedra each, resulting in a formula of Be3F9. In the tetrafluoroberyllates, the tetrahedra can rotate to various degrees. At room temperature, they are hindered from moving. But as temperature increases, they can rotate around the threefold axis, (ie a line through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrafluoroammonium
The tetrafluoroammonium cation (also known as perfluoroammonium) is a positively charged polyatomic ion with chemical formula . It is equivalent to the ammonium ion where the hydrogen atoms surrounding the central nitrogen atom have been replaced by fluorine. Tetrafluoroammonium ion is isoelectronic with tetrafluoromethane , trifluoramine oxide and the tetrafluoroborate anion. The tetrafluoroammonium ion forms salts with a large variety of fluorine-bearing anions. These include the bifluoride anion (), tetrafluorobromate (), metal pentafluorides ( where M is Ge, Sn, or Ti), hexafluorides ( where M is P, As, Sb, Bi, or Pt), heptafluorides ( where M is W, U, or Xe), octafluorides (), various oxyfluorides ( where M is W or U; , ), and perchlorate (). Attempts to make the nitrate salt, , were unsuccessful because of quick fluorination: + → + . Structure The geometry of the tetrafluoroammonium ion is tetrahedral, with an estimated nitrogen-fluorine bond length of 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen borate or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salts, and can react with alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxoacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However boric acid and borates have been used since the time of the ancient Greeks for cleaning, preserving food, and other activities. Molecular a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . It is an octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the hexafluorosilicate dianion, , and hexafluoroantimonate . In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion. Synthesis Hexafluorophosphate salts can be prepared by the reaction of phosphorus pentachloride and alkali or ammonium halide in a solution of hydrofluoric acid: : Hexafluorophosphoric acid can be prepared by direct reaction of hydrogen fluoride with phosphorus pentafluoride. It is a strong Brønsted acid that is typically generated ''in situ'' immediately before its use. : These reactions require specialized equipment to safely handle the hazards associated with hydrofluoric acid and hydrogen fluoride. Quantitative analysis Several methods of quantitative analysis for the hexafluorophosphate ion have been de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formula un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher than water. On Earth, natural rubidium comprises two isotopes: 72% is a stable isotope 85Rb, and 28% is slightly radioactive 87Rb, with a half-life of 48.8 billion years—more than three times as long as the estimated age of the universe. German chemists Robert Bunsen and Gustav Kirchhoff discovered rubidium in 1861 by the newly developed technique, flame spectroscopy. The name comes from the Latin word , meaning deep red, the color of its emission spectrum. Rubidium's compounds have various chemical and electronic applications. Rubidium metal is easily vaporized and has a convenient spectral absorption range, making it a frequent target for laser manipulation of atoms. Rubidium is not a known nutrient for any living organisms. However, r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkali Metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names for the elements in some languages, such as German and Russian. rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute Group (periodic table)#Group names, group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an atomic orbital, s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of periodic trends, group trends in properties in the periodic table, with elements exhibiting well-characterised homology (chemistry), homologous behaviour. This family of elements is also known as the lithium family after its leading element. The alkali metals are all sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



4.png)



_chloride.jpg)

