|
Fibrous Protein
In molecular biology, fibrous proteins or scleroproteins are one of the three main classifications of protein structure (alongside globular and membrane proteins). Fibrous proteins are made up of elongated or fibrous polypeptide chains which form filamentous and sheet-like structures. These kind of protein can be distinguished from globular protein by its low solubility in water. Such proteins serve protective and structural roles by forming connective tissue, tendons, bone matrices, and muscle fiber. Fibrous proteins consist of many superfamilies including keratin, collagen, elastin, and fibrin. Collagen is the most abundant of these proteins which exists in vertebrate connective tissue including tendon, cartilage, and bone. Biomolecular structure A fibrous protein forms long protein filaments, which are shaped like rods or wires. Fibrous proteins are structural or storage proteins that are typically inert and water-insoluble. A fibrous protein occurs as an aggregat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
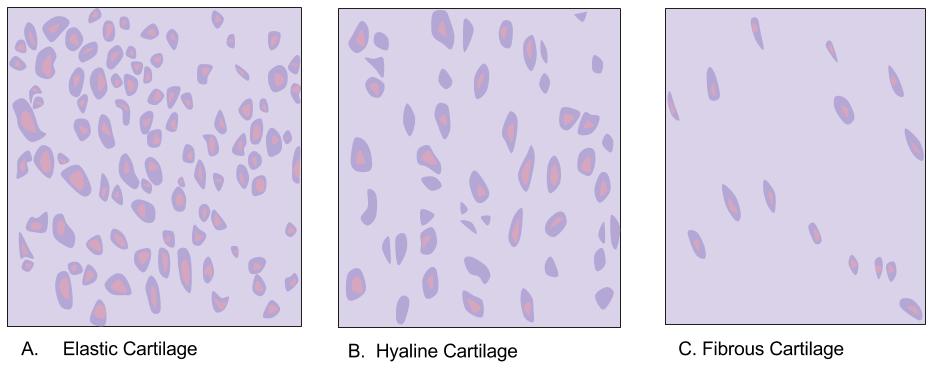
Cartilage
Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin. Because of its rigidity, cartilage often serves the purpose of holding tubes open in the body. Examples include the rings of the trachea, such as the cricoid cartilage and carina. Cartilage is composed of specialized cells called chondrocytes that produce a large amount of collagenous extracellular matrix, abundant ground substance that is rich in pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-links
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen Helix
In molecular biology, the collagen triple helix or type-2 helix is the main secondary structure of various types of fibrous collagen, including type I collagen. In 1954, Ramachandran & Kartha (13, 14) advanced a structure for the collagen triple helix on the basis of fiber diffraction data. It consists of a triple helix made of the repetitious amino acid sequence glycine-X-Y, where X and Y are frequently proline or hydroxyproline. Collagen folded into a triple helix is known as tropocollagen. Collagen triple helices are often bundled into fibrils which themselves form larger fibres, as in tendons. Structure Glycine, proline, and hydroxyproline must be in their designated positions with the correct configuration. For example, hydroxyproline in the Y position increases the thermal stability of the triple helix, but not when it is located in the X position. The thermal stabilization is also hindered when the hydroxyl group has the wrong configuration. Due to the high abundance of g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Structure
Protein secondary structure is the three dimensional conformational isomerism, form of ''local segments'' of proteins. The two most common Protein structure#Secondary structure, secondary structural elements are alpha helix, alpha helices and beta sheets, though beta turns and omega loops occur as well. Secondary structure elements typically spontaneously form as an intermediate before the protein protein folding, folds into its three dimensional protein tertiary structure, tertiary structure. Secondary structure is formally defined by the pattern of hydrogen bonds between the Amine, amino hydrogen and carboxyl oxygen atoms in the peptide backbone chain, backbone. Secondary structure may alternatively be defined based on the regular pattern of backbone Dihedral angle#Dihedral angles of proteins, dihedral angles in a particular region of the Ramachandran plot regardless of whether it has the correct hydrogen bonds. The concept of secondary structure was first introduced by Kaj Ulrik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Residue (chemistry)
In chemistry, residue is whatever remains or acts as a contaminant after a given class of events. Residue may be the material remaining after a process of preparation, separation, or purification, such as distillation, evaporation, or filtration. It may also denote the undesired by-products of a chemical reaction. Food safety Toxic chemical residues, wastes or contamination from other processes, are a concern in food safety. For example, the U.S. Food and Drug Administration (FDA) and the Canadian Food Inspection Agency (CFIA) have guidelines for detecting chemical residues that are possibly dangerous to consume. Characteristic units within a molecule ''Residue'' may refer to an atom or a group of atoms that forms part of a molecule, such as a methyl group. Biochemistry In biochemistry and molecular biology, a residue refers to a specific monomer within the polymeric chain of a polysaccharide, protein or nucleic acid. One might say, "This protein consists of 118 amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide Sequence
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pepti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Side Chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a larger hydrocarbon backbone. It is one factor in determining a molecule's properties and reactivity. A side chain is also known as a pendant chain, but a pendant group (side group) has a different definition. Conventions The placeholder R is often used as a generic placeholder for alkyl (saturated hydrocarbon) group side chains in chemical structure diagrams. To indicate other non-carbon groups in structure diagrams, X, Y, or Z are often used. History The ''R'' symbol was introduced by 19th-century French chemist Charles Frédéric Gerhardt, who advocated its adoption on the grounds that it would be widely recognizable and intelligible given its correspondence in multiple European languages to the initial letter of "root" or "residue": ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. Hydrophobic is often used interchangeably with lipophilic, "fat-loving". However, the two terms are not synonymous. While hydrophobic substances are usually lipophilic, there are exceptions, suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemically Inert
In chemistry, the term chemically inert is used to describe a substance that is not chemically reactive. From a thermodynamic perspective, a substance is inert, or nonlabile, if it is thermodynamically unstable (positive standard Gibbs free energy of formation) yet decomposes at a slow, or negligible rate. Most of the noble gases, which appear in the last column of the periodic table, are classified as inert (or unreactive). These elements are stable in their naturally occurring form (gaseous form) and they are called inert gases. Noble gas The noble gases (helium, neon, argon, krypton, xenon and radon) were previously known as 'inert gases' because of their perceived lack of participation in any chemical reactions. The reason for this is that their outermost electron shells (valence shells) are completely filled, so that they have little tendency to gain or lose electrons. They are said to acquire a noble gas configuration, or a full electron configuration. It is now ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |