|
Fluorosulfonate
Fluorosulfonate, in organic chemistry, is a functional group that has the chemical formula F-SO2-R, and typically is a very good leaving group. In organic chemistry, fluorosulfonate is different than fluorosulfate. In fluorosulfonates, sulfur atom is directly bonded to a non-oxygen atom such as carbon. In inorganic chemistry, fluorosulfonate is another term for fluorosulfate, the anion F-SO2-O−, the conjugate base of fluorosulfonic acid. They form a series of salts with metal and organic cations called fluorosulfates. Organic (alkyl) fluorosulfonates are usually strong alkylation agents, similar to triflate esters (F3C-SO2-OR). But unlike the triflate group, the fluorosulfonate group is not stable against hydrolysis. Therefore, fluorosulfonate esters are less frequently used as alkylation agents than triflate esters. See also * Fluorosulfite * Methyl fluorosulfonate Methyl fluorosulfonate, also known as magic methyl, is the organic compound with the formula FSO2OCH3. It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorosulfonate Group
Fluorosulfonate, in organic chemistry, is a functional group that has the chemical formula F-SO2-R, and typically is a very good leaving group. In organic chemistry, fluorosulfonate is different than fluorosulfate. In fluorosulfonates, sulfur atom is directly bonded to a non-oxygen atom such as carbon. In inorganic chemistry, fluorosulfonate is another term for fluorosulfate, the anion F-SO2-O−, the conjugate base of fluorosulfonic acid. They form a series of salts with metal and organic cations called fluorosulfates. Organic (alkyl) fluorosulfonates are usually strong alkylation agents, similar to triflate esters (F3C-SO2-OR). But unlike the triflate group, the fluorosulfonate group is not stable against hydrolysis. Therefore, fluorosulfonate esters are less frequently used as alkylation agents than triflate esters. See also * Fluorosulfite * Methyl fluorosulfonate References Functional groups Leaving groups Sulfonates {{chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorosulfates
The fluorosulfates or fluorosulfonates are a set of salts of fluorosulfuric acid with an ion formula SO3F−. The fluorosulfate anion can be treated as though it were a hydrogen sulfate anion with hydroxyl substituted by fluorine. The fluorosulfate ion has a low propensity to form complexes with metal cations. Since fluorine is similar in size to oxygen, the fluorosulfate ion is roughly tetrahedral and forms salts similar to those of the perchlorate ion. It is isoelectronic with hydrogen sulfate, . When an organic group is substituted for the anions, organic fluorosulfonates are formed. In solution the fluorosulfate anion is completely ionised. The volume of the ions is 47.8 cm3/mol. Most metal ions, and quaternary ammonium ions, can form fluorosulfate salts. Different ways to make these salts include treating a metal chloride with anhydrous fluorosulfuric acid, which releases hydrogen chloride gas. Double decomposition methods utilising a metal sulfate with barium fluorosulfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorosulfate
Chlorosulfuric acid (IUPAC name: sulfurochloridic acid) is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid, being the sulfonic acid of chlorine. It is a distillable, colorless liquid which is hygroscopic and a powerful lachrymator. Commercial samples usually are pale brown or straw colored. Salts and esters of chlorosulfuric acid are known as chlorosulfates. Structure and properties Chlorosulfuric acid is a tetrahedral molecule. The formula is more descriptively written SO2(OH)Cl, but HSO3Cl is traditional. It is an intermediate, chemically and conceptually, between sulfuryl chloride (SO2Cl2) and sulfuric acid (H2SO4). The compound is rarely obtained pure. Upon standing with excess sulfur trioxide, it decomposes to pyrosulfuryl chlorides: :2 ClSO3H + SO3 → H2SO4 + S2O5Cl2 Synthesis The industrial synthesis entails the reaction of hydrogen chloride with a solution of sulfur trioxide in sulfuric acid: :HCl + SO3 → ClSO3H It can als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Fluorosulfonate
Methyl fluorosulfonate, also known as magic methyl, is the organic compound with the formula FSO2OCH3. It is a colorless liquid that is used as a strong methylating agent in organic synthesis. Because of its extreme toxicity, it has largely been replaced by the related reagent methyl trifluoromethanesulfonate. Synthesis and reactions It is prepared by distillation of an equimolar mixture of fluorosulfonic acid and dimethyl sulfate. It was originally produced by the reaction of methanol with fluorosulfonic acid. Methyl fluorosulfonate is a highly electrophilic reagent for methylation. It is ranked as less powerful than methyl trifluoromethanesulfonate. Toxicity Similar to phosgene, it is acutely toxic by inhalation, with an LC50 (rat, 1 hour) of about 5 ppm. Several cases of poisoning resulting in death from pulmonary edema Pulmonary edema, also known as pulmonary congestion, is excessive edema, liquid accumulation in the parenchyma, tissue and pulmonary alveolus, ai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorosulfite
Fluorosulfite is an ion with the formula SO2F−. The term is also used for compounds or salts containing this group. Fluorosulfite was discovered in 1953 by F Seel and H Meier. Organic compounds with the name "fluorosulfite" contain the group -OS(O)F. Preparation (CH3)2N)3SOSO2F] can be prepared from Thionyl tetrafluoride, OSF4 and Me3SiNMe2. Alkali metal fluorosulfites can be made by soaking the metal fluoride in liquid sulfur dioxide for a few days. β-CsSO2F converts to α-CsSO2F when heated to 110 °C for a couple of days but remains stable below 50 °C. Properties The fluorosulfite ion is tetrahedral, with sulfur at the top. The oxygen to sulfur bonds are 147.8 pm and the fluorine to sulfur bond is >169.0 pm long. In solid ionic fluorosulfites, the ion is not fixed in orientation and continuously turns around resulting in dynamic disorder. At room temperature this turning rate is from 2×105 to 107 Hz. When cooled the rate of rotation slows, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflate
In organic chemistry, triflate (systematic name: trifluoromethanesulfonate), is a functional group with the formula and structure . The triflate group is often represented by , as opposed to −Tf, which is the triflyl group, . For example, ''n''-butyl triflate can be written as . The corresponding triflate anion, , is an extremely stable polyatomic ion; this comes from the fact that triflic acid () is a superacid; i.e. it is more acidic than pure sulfuric acid, already one of the strongest acids known. Applications A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution, Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in SN2 reactions, they must be stored in conditions free of nucleophiles (such as water). The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to other molecular fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorosulfonic Acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula HSO3F. It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, H2SO4, substituting a fluorine atom for one of the hydroxyl groups. It is a colourless liquid, although commercial samples are often yellow.Erhardt Tabel, Eberhard Zirngiebl, Joachim Maas "Fluorosulfuric Acid" in "Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. Chemical properties Fluorosulfuric acid is a free-flowing colorless liquid. It is soluble in polar organic solvents (e.g. nitrobenzene, acetic acid, and ethyl acetate), but poorly soluble in nonpolar solvents such as alkanes. Reflecting its strong acidity, it dissolves almost all organic compounds that are even weak proton acceptors. HSO3F hydrolyzes slowly to hydrogen fluoride (HF) and sulfuric acid. The related triflic acid () retains the high acidity of HSO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine Fluorosulfonate
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
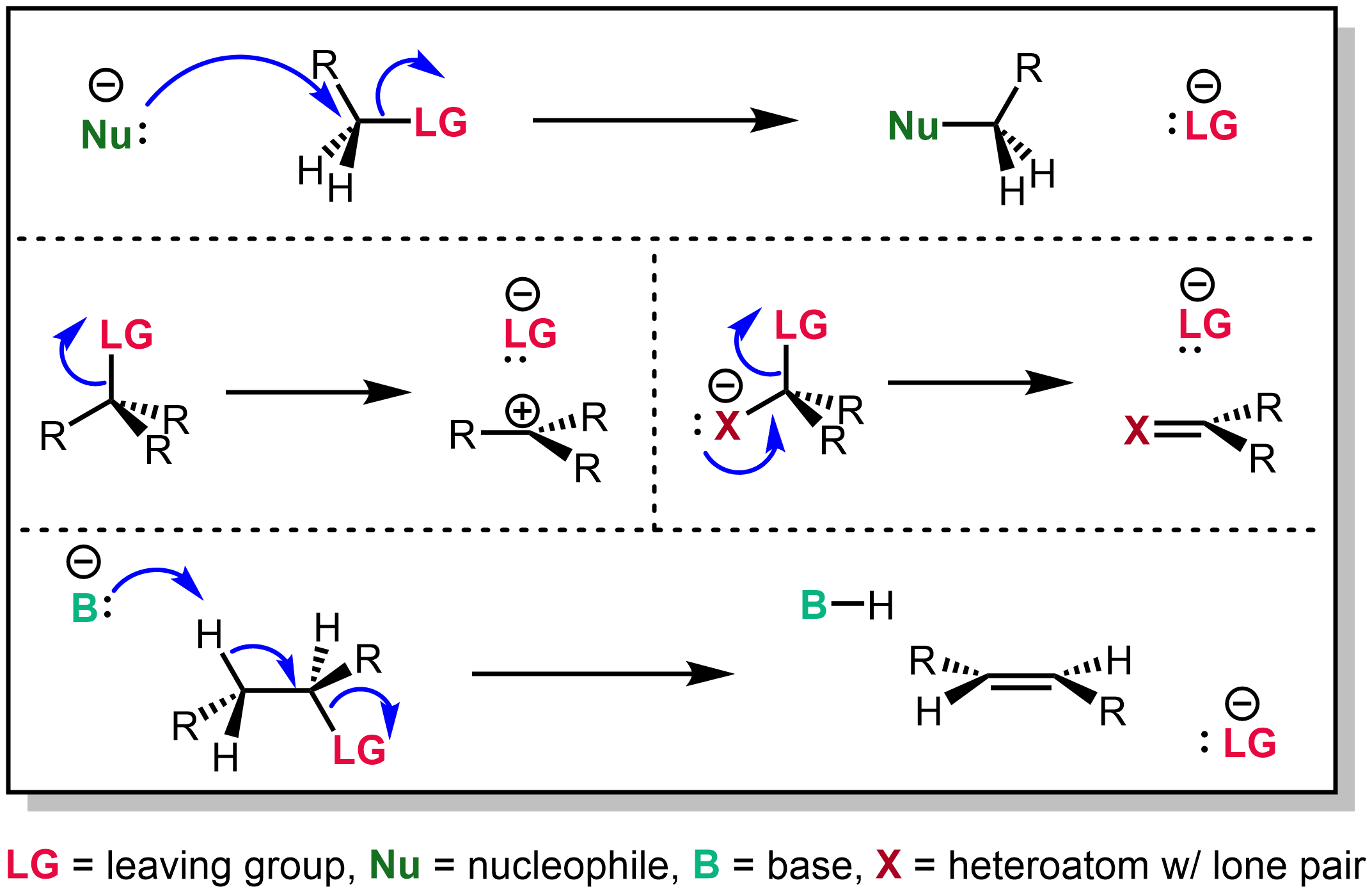
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |