|
Fluorometer
A fluorometer, fluorimeter or fluormeter is a device used to measure parameters of visible spectrum fluorescence: its intensity and wavelength distribution of emission spectrum after excitation by a certain spectrum of light. These parameters are used to identify the presence and the amount of specific molecules in a medium. Modern fluorometers are capable of detecting fluorescent molecule concentrations as low as 1 part per trillion. Fluorescence analysis can be orders of magnitude more sensitive than other techniques. Applications include chemistry/biochemistry, medicine, environmental monitoring. For instance, they are used to measure chlorophyll fluorescence to investigate plant physiology. Components and Design Typically fluorometers utilize a double beam. These two beams work in tandem to decrease the noise created from radiant power fluctuations. The upper beam is passed through a filter or monochromator and passes through the sample. The lower beam is passed through an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorimeter
A fluorometer, fluorimeter or fluormeter is a device used to measure parameters of visible spectrum fluorescence: its intensity and wavelength distribution of emission spectrum after excitation by a certain spectrum of light. These parameters are used to identify the presence and the amount of specific molecules in a medium. Modern fluorometers are capable of detecting fluorescent molecule concentrations as low as 1 part per trillion. Fluorescence analysis can be orders of magnitude more sensitive than other techniques. Applications include chemistry/biochemistry, medicine, environmental monitoring. For instance, they are used to measure chlorophyll fluorescence to investigate plant physiology. Components and Design Typically fluorometers utilize a double beam. These two beams work in tandem to decrease the noise created from radiant power fluctuations. The upper beam is passed through a filter or monochromator and passes through the sample. The lower beam is passed through an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
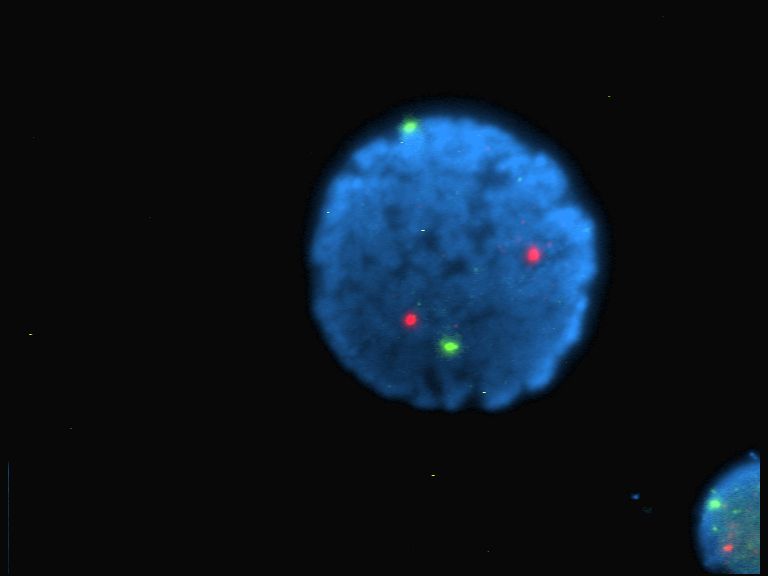
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll Fluorescence
Chlorophyll fluorescence is light re-emitted by chlorophyll molecules during return from excited to non-excited states. It is used as an indicator of photosynthetic energy conversion in plants, algae and bacteria. Excited chlorophyll dissipates the absorbed light energy by driving photosynthesis (photochemical energy conversion), as heat in non-photochemical quenching or by emission as fluorescence radiation. As these processes are complementary processes, the analysis of chlorophyll fluorescence is an important tool in plant research with a wide spectrum of applications. The Kautsky effect Upon illumination of a dark-adapted leaf, there is a rapid rise in fluorescence from Photosystem II (PSII), followed by a slow decline. First observed by ''Kautsky et al., 1932'', this is called the Kautsky Effect. This variable rise in chlorophyll fluorescence rise is due to photosystem II. Fluorescence from photosystem I is not variable, but constant. The increase in fluorescence is du ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorophore
A fluorophore (or fluorochrome, similarly to a chromophore) is a fluorescent chemical compound that can re-emit light upon light excitation. Fluorophores typically contain several combined aromatic groups, or planar or cyclic molecules with several π bonds. Fluorophores are sometimes used alone, as a tracer in fluids, as a dye for staining of certain structures, as a substrate of enzymes, or as a probe or indicator (when its fluorescence is affected by environmental aspects such as polarity or ions). More generally they are covalently bonded to a macromolecule, serving as a marker (or dye, or tag, or reporter) for affine or bioactive reagents (antibodies, peptides, nucleic acids). Fluorophores are notably used to stain tissues, cells, or materials in a variety of analytical methods, i.e., fluorescent imaging and spectroscopy. Fluorescein, via its amine-reactive isothiocyanate derivative fluorescein isothiocyanate (FITC), has been one of the most popular fluorophores. Fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemiluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction. There may also be limited emission of heat. Given reactants A and B, with an excited intermediate ◊, : + -> lozenge -> roducts+ light For example, if is luminol and is hydrogen peroxide in the presence of a suitable catalyst we have: :\underset + \underset -> 3-APAlozenge-> + light where: * 3-APA is 3-aminophthalate * 3-APA ''◊is the vibronic excited state fluorescing as it decays to a lower energy level. General description The decay of this excited state ''◊to a lower energy level causes light emission. In theory, one photon of light should be given off for each molecule of reactant. This is equivalent to the Avogadro number of photons per mole of reactant. In actual practice, non-enzymatic reactions seldom exceed 1% QC, quantum efficiency. In a chemical reaction, reactants collide to form a transition state, the enthalpic maximum in a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an Insulator (electricity), electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pasteurization
Pasteurization or pasteurisation is a process of food preservation in which packaged and non-packaged foods (such as milk and fruit juices) are treated with mild heat, usually to less than , to eliminate pathogens and extend shelf life. The process is intended to destroy or deactivate microorganisms and enzymes that contribute to food spoilage or risk of disease, including vegetative bacteria, but most bacterial spores survive the process. The process is named after the French microbiologist Louis Pasteur whose research in the 1860s demonstrated that thermal processing would deactivate unwanted microorganisms in wine. Spoilage enzymes are also inactivated during pasteurization. Today, pasteurization is used widely in the dairy industry and other food processing industries to achieve food preservation and food safety. By the year 1999, most liquid products were heat treated in a continuous system where heat can be applied using a plate heat exchanger or the direct or indirec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionization, ionize atoms, it can cause chemical reactions and causes many substances to glow or fluorescence, fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Denaturation (biochemistry)
In biochemistry, denaturation is a process in which proteins or nucleic acids lose the quaternary structure, tertiary structure, and secondary structure which is present in their native state, by application of some external stress or compound such as a strong acid or base, a concentrated inorganic salt, an organic solvent (e.g., alcohol or chloroform), agitation and radiation or heat. If proteins in a living cell are denatured, this results in disruption of cell activity and possibly cell death. Protein denaturation is also a consequence of cell death. Denatured proteins can exhibit a wide range of characteristics, from conformational change and loss of solubility to aggregation due to the exposure of hydrophobic groups. The loss of solubility as a result of denaturation is called ''coagulation''. Denatured proteins lose their 3D structure and therefore cannot function. Protein folding is key to whether a globular or membrane protein can do its job correctly; it must be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioflavin
Thioflavins are fluorescent dyes that are available as at least two compounds, namely Thioflavin T and Thioflavin S. Both are used for histology staining and biophysics, biophysical studies of protein aggregation. In particular, these dyes have been used since 1989 to investigate amyloid formation. They are also used in biophysical studies of the electrophysiology of bacteria. Thioflavins are corrosive, Irritation, irritants, and are acutely toxic, causing serious eye damage. Thioflavin T has been used in research into Alzheimer's disease and other neurodegenerative diseases. Thioflavin T Thioflavin T (Basic Yellow 1, Methylene yellow, CI 49005, or ThT) is a benzothiazole salt obtained by the methylation of dehydrothiotoluidine with methanol in the presence of hydrochloric acid. The dye is widely used to visualize and quantify the presence of misfolded protein aggregates called amyloid, both ''in vitro'' and ''in vivo'' (e.g., Senile plaques, plaques composed of amyloid beta found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |