|
Flame Ionization Detector
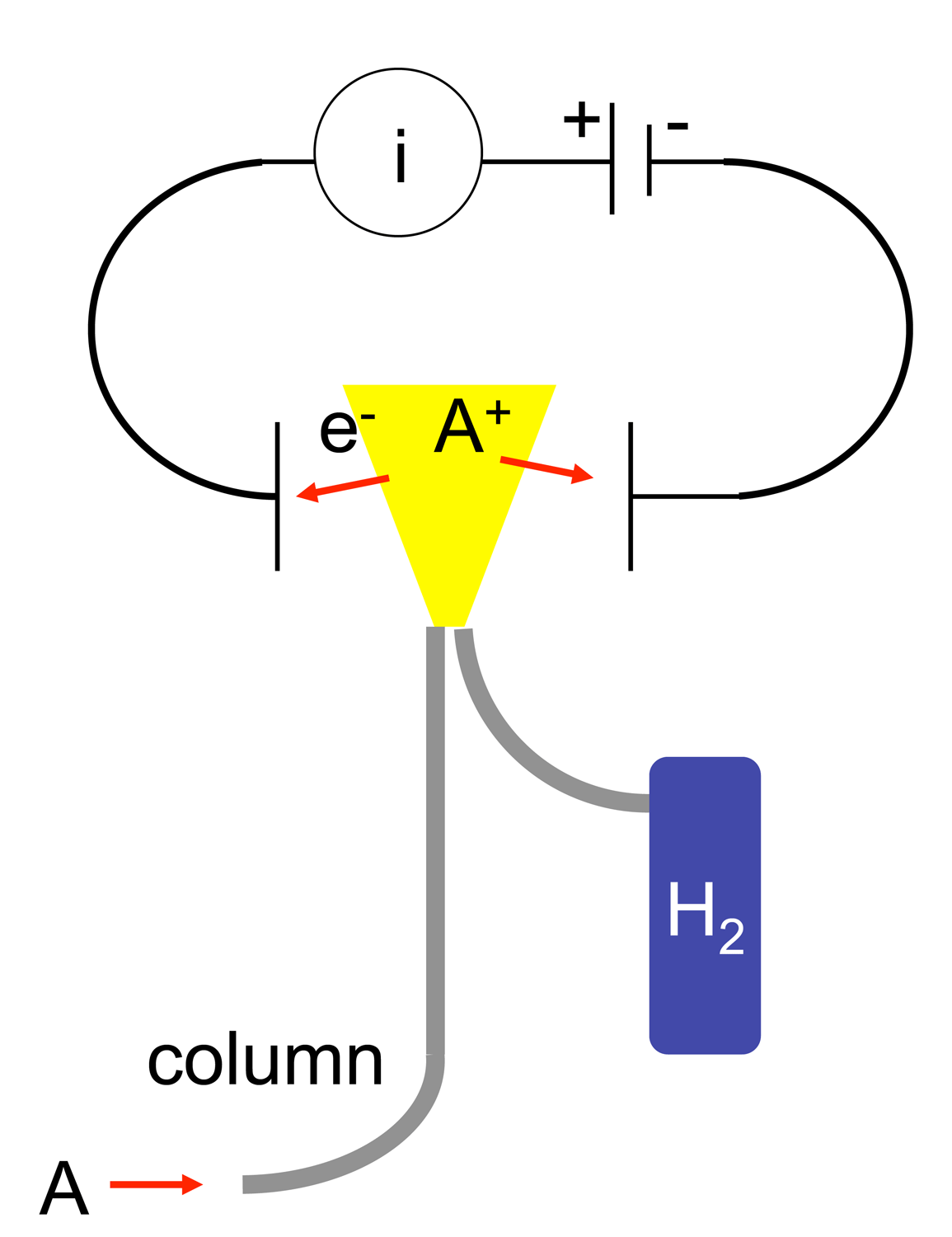
A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ion per unit time make this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring, fugitive emissions monitoring and internal combustion engine emissions measurement in stationary or portable instruments. History The first flame ionization detectors were developed simultaneously and independently in 1957 by McWilliam and Dewar at Imperial Chemical Industries of Australia and New Zealand (ICIANZ, see Orica history) Central Research Laboratory, Ascot Vale, Melbourne, Australia. and by Harley and Pretorius at the University of Pretoria in Pretoria, South Africa. In 1959, Perkin Elmer Corp. included a flame ionization detector in its Vapor Fractometer. Operating principle The operation of the FID is based on the detection of ions formed dur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame Ionization Detector Schematic
A flame (from Latin '' flamma'') is the visible, gaseous part of a fire. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionized gaseous components of sufficient density they are then considered plasma. Mechanism Color and temperature of a flame are dependent on the type of fuel involved in the combustion, as, for example, when a lighter is held to a candle. The applied heat causes the fuel molecules in the candle wax to vaporize (if this process happens in inert atmosphere without oxidizer, it is called pyrolysis). In this state they can then readily react with oxygen in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame. The high temperature of the flame causes the vaporized fuel molecules to decompose, forming various incomplete combustion products and free radicals, and these products then react with each other and with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrator
An integrator in measurement and control applications is an element whose output signal is the time integral of its input signal. It accumulates the input quantity over a defined time to produce a representative output. Integration is an important part of many engineering and scientific applications. Mechanical integrators are the oldest application, and are still used in such as metering of water flow or electric power. Electronic analogue integrators are the basis of analog computer An analog computer or analogue computer is a type of computer that uses the continuous variation aspect of physical phenomena such as electrical, mechanical, or hydraulic quantities (''analog signals'') to model the problem being solved. ...s and charge amplifiers. Integration is also performed by digital computing algorithms. In signal processing circuits :''See also Operational amplifier applications#Integration and differentiation, Integrator at op amp applications'' An electronics, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen. The stationary phase is a microscopic lay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity Detector
The thermal conductivity detector (TCD), also known as a katharometer, is a bulk property detector and a chemical specific detector commonly used in gas chromatography. This detector senses changes in the thermal conductivity of the column eluent and compares it to a reference flow of carrier gas. Since most compounds have a thermal conductivity much less than that of the common carrier gases of helium or hydrogen, when an analyte elutes from the column the effluent thermal conductivity is reduced, and a detectable signal is produced. Operation The TCD consists of an electrically heated filament in a temperature-controlled cell. Under normal conditions there is a stable heat flow from the filament to the detector body. When an analyte elutes and the thermal conductivity of the column effluent is reduced, the filament heats up and changes resistance. This resistance change is often sensed by a Wheatstone bridge circuit which produces a measurable voltage change. The column effluent fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame Detector
A flame detector is a sensor designed to detect and respond to the presence of a flame or fire, allowing flame detection. Responses to a detected flame depend on the installation, but can include sounding an alarm, deactivating a fuel line (such as a propane or a natural gas line), and activating a fire suppression system. When used in applications such as industrial furnaces, their role is to provide confirmation that the furnace is working properly; it can be used to turn off the ignition system though in many cases they take no direct action beyond notifying the operator or control system. A flame detector can often respond faster and more accurately than a smoke or heat detector due to the mechanisms it uses to detect the flame. Optical flame detectors Ultraviolet detector Ultraviolet (UV) detectors work by detecting the UV radiation emitted at the instant of ignition. While capable of detecting fires and explosions within 3–4 milliseconds, a time delay of 2–3 seconds is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyarc Reactor
The Polyarc reactor is a scientific tool for the measurement of organic molecules. It is paired with a flame ionization detector (FID) in a gas chromatograph (GC) to improve the sensitivity of the FID and give a uniform detector response for all organic molecules (GC-Polyarc/FID). The reactor converts the carbon atoms of organic molecules in GC column effluents into methane before reaching the FID. The resulting detector response is uniform on a per carbon basis and allows the FID to have a good carbon sensitivity. GC-Polyarc/FID peak areas (integrated detector responses) are equivalent on a per carbon basis, thus eliminating the need for response factors and calibration standards. The GC-Polyarc/FID method also improves the response of the FID to a number of molecules with poor/low response including, carbon monoxide (CO), carbon dioxide (CO2), carbon disulfide (CS2), carbonyl sulfide (COS), hydrogen cyanide (HCN), formamide (CH3NO), formaldehyde (CH2O) and formic acid (CH2O2), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanizer
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which converts CO2 and CO into methane CH4. Methanizers contain a hydrogenation catalyst to achieve this conversion. Nickel is commonly used as the catalyst and there are alternatives available. Chemistry On-line catalytic reduction of carbon monoxide to methane for detection by FID was described by Porter & Volman, who suggested that both carbon dioxide and carbon monoxide could also be converted to methane with the same nickel catalyst. This was confirmed by Johns & Thompson, who determined optimum operating parameters for each of the gases. CO2 + 2H2 ↔ CH4 + O2 2CO + 4H2 ↔ 2CH4 + O2 Typical design The catalyst traditionally consists of a 2% coating of Ni in the form of nickel nitrate deposited on a chromatographic packing mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
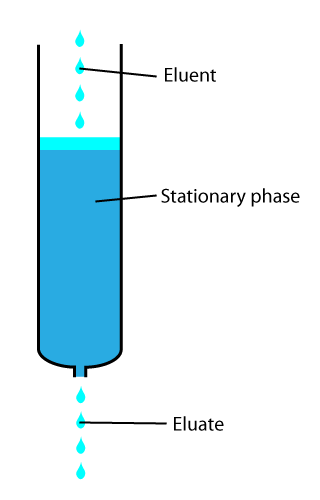
Elution
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent; as in washing of loaded ion-exchange resins to remove captured ions. In a liquid chromatography experiment, for example, an analyte is generally adsorbed, or "bound to", an adsorbent in a liquid chromatography column. The adsorbent, a solid phase (stationary phase), is a powder which is coated onto a solid support. Based on an adsorbent's composition, it can have varying affinities to "hold" onto other molecules—forming a thin film on the surface of its particles. Elution then is the process of removing analytes from the adsorbent by running a solvent, called an "eluent", past the adsorbent/analyte complex. As the solvent molecules "elute", or travel down through the chromatography column, they can either pass by the adsorbent/analyte complex or they can displace the analyte by binding to the adsorbent in its place. After the solvent molecules displac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flame Ionization Detector
A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ion per unit time make this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring, fugitive emissions monitoring and internal combustion engine emissions measurement in stationary or portable instruments. History The first flame ionization detectors were developed simultaneously and independently in 1957 by McWilliam and Dewar at Imperial Chemical Industries of Australia and New Zealand (ICIANZ, see Orica history) Central Research Laboratory, Ascot Vale, Melbourne, Australia. and by Harley and Pretorius at the University of Pretoria in Pretoria, South Africa. In 1959, Perkin Elmer Corp. included a flame ionization detector in its Vapor Fractometer. Operating principle The operation of the FID is based on the detection of ions formed dur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ASTM
ASTM International, formerly known as American Society for Testing and Materials, is an international standards organization that develops and publishes voluntary consensus technical standards for a wide range of materials, products, systems, and services. Some 12,575 ASTM voluntary consensus standards operate globally. The organization's headquarters is in West Conshohocken, Pennsylvania, about northwest of Philadelphia. It is founded in 1902 as the American Section of the International Association for Testing Materials (see also International Organization for Standardization). History A group of scientists and engineers, led by Charles Dudley, formed ASTM in 1898 to address the frequent rail breaks affecting the fast-growing railroad industry. The group developed a standard for the steel used to fabricate rails. Originally called the "American Society for Testing Materials" in 1902, it became the "American Society for Testing And Materials" in 1961. In 2001, ASTM off ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-and-flame-sensor.jpg)