|
Fibulin-1
FBLN1 is the gene encoding fibulin-1, an extracellular matrix and plasma protein. Function Fibulin-1 is a secreted glycoprotein that is found in association with extracellular matrix structures including fibronectin-containing fibers, elastin-containing fibers and basement membranes. Fibulin-1 binds to a number of extracellular matrix constituents including fibronectin, nidogen-1, and the proteoglycan, versican. Fibulin-1 is also a blood protein capable of binding to fibrinogen. Structure Fibulin-1 has modular domain structure and includes a series of nine epidermal growth factor-like modules followed by a fibulin-type module, a module found in all members of the fibulin gene family. The human fibulin-1 gene, FBLN1, encodes four splice variants designated fibulin-1A, B, C and D, which differ in their carboxy terminal regions. In mouse, chicken and the nematode, '' C. elegans'', only two fibulin-1 variants are produced, fibulin-1C and fibulin-1D. Interactions FBLN1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibulin
Fibulin (FY-beau-lin) (now known as Fibulin-1 FBLN1) is the prototypic member of a multigene family, currently with seven members. Fibulin-1 is a calcium-binding glycoprotein. In vertebrates, fibulin-1 is found in blood and extracellular matrices. In the extracellular matrix, fibulin-1 associates with basement membranes and elastic fibers. The association with these matrix structures is mediated by its ability to interact with numerous extracellular matrix constituents including fibronectin, proteoglycans, laminins and tropoelastin. In blood, fibulin-1 binds to fibrinogen and incorporates into clots. Fibulins are secreted glycoproteins that become incorporated into a fibrillar extracellular matrix when expressed by cultured cells or added exogenously to cell monolayers. The five known members of the family share an elongated structure and many calcium-binding sites, owing to the presence of tandem arrays of epidermal growth factor-like domains. They have overlapping binding si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amyloid Precursor Protein
Amyloid-beta precursor protein (APP) is an integral membrane protein expressed in many biological tissue, tissues and concentrated in the synapses of neurons. It functions as a cell surface receptor and has been implicated as a regulator of synapse formation, neural plasticity, antimicrobial activity, and iron export. It is coded for by the gene ''APP'' and regulated by substrate presentation. APP is best known as the precursor molecule whose proteolysis generates amyloid beta (Aβ), a polypeptide containing 37 to 49 amino acid residues, whose Amyloid#Structure, amyloid fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. Genetics Amyloid-beta precursor protein is an ancient and highly Conserved sequence, conserved protein. In humans, the gene ''APP'' is located on chromosome 21 and contains 18 exons spanning 290 kilobases. Several alternative splicing isoforms of APP have been observed in humans, ranging in len ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epidermal Growth Factor
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-k Da and has 53 amino acid residues and three intramolecular disulfide bonds. EGF was originally described as a secreted peptide found in the submaxillary glands of mice and in human urine. EGF has since been found in many human tissues, including platelets, submandibular gland (submaxillary gland), and parotid gland. Initially, human EGF was known as urogastrone. Structure In humans, EGF has 53 amino acids (sequence NSDSECPLSHDGYCLHDGVCMYIEALDKYACNCVVGYIGERCQYRDLKWWELR), with a molecular mass of around 6 kDa. It contains three disulfide bridges (Cys6-Cys20, Cys14-Cys31, Cys33-Cys42). Function EGF, via binding to its cognate receptor, results in cellular proliferation, differentiation, and survival. Salivary EGF, which seems to be regulated by dietary inorganic iodine, also plays an important physiological role in the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NOV (gene)
NOV (nephroblastoma overexpressed) also known as CCN3 is a matricellular protein that in humans is encoded by the ''NOV'' gene. CCN family NOV is a member of the CCN family of secreted, extracellular matrix (ECM)-associated signaling proteins (see also CCN intercellular signaling protein). The CCN acronym is derived from the first three members of the family being identified, namely CYR61 (cysteine-rich angiogenic inducer 61, or CCN1), CTGF (connective tissue growth factor, or CCN2), and NOV. These proteins, together with WISP1 (CCN4), WISP2 (CCN5), and WISP3 (CCN6) comprise the six-member CCN family in vertebrates and have been renamed CCN1-6 in the order of their discovery by international consensus. Structure The human NOV protein contains 357 amino acids with an N-terminal secretory signal peptide followed by four structurally distinct domains with homologies to insulin-like growth factor binding protein (IGFBP), von Willebrand type C repeats ( vWC), thrombospondin type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caenorhabditis Elegans
''Caenorhabditis elegans'' () is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a blend of the Greek ''caeno-'' (recent), ''rhabditis'' (rod-like) and Latin ''elegans'' (elegant). In 1900, Maupas initially named it ''Rhabditides elegans.'' Osche placed it in the subgenus ''Caenorhabditis'' in 1952, and in 1955, Dougherty raised ''Caenorhabditis'' to the status of genus. ''C. elegans'' is an unsegmented pseudocoelomate and lacks respiratory or circulatory systems. Most of these nematodes are hermaphrodites and a few are males. Males have specialised tails for mating that include spicules. In 1963, Sydney Brenner proposed research into ''C. elegans,'' primarily in the area of neuronal development. In 1974, he began research into the molecular and developmental biology of ''C. elegans'', which has since been extensively used as a model organism. It was the first multice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
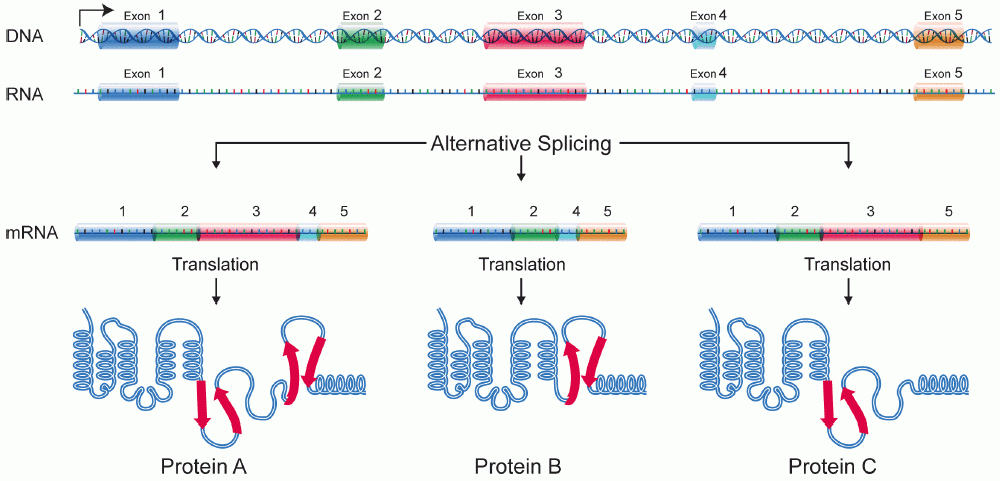
Alternative Splicing
Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be included within or excluded from the final, processed messenger RNA (mRNA) produced from that gene. This means the exons are joined in different combinations, leading to different (alternative) mRNA strands. Consequently, the proteins translated from alternatively spliced mRNAs will contain differences in their amino acid sequence and, often, in their biological functions (see Figure). Biologically relevant alternative splicing occurs as a normal phenomenon in eukaryotes, where it increases the number of proteins that can be encoded by the genome. In humans, it is widely believed that ~95% of multi-exonic genes are alternatively spliced to produce functional alternative products from the same gene but many scientists believe that most o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoprotein
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. Each type of conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Versican
Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the ''VCAN'' gene. Versican is a large chondroitin sulfate proteoglycan with an apparent molecular mass of more than 1000kDa. In 1989, Zimmermann and Ruoslahti cloned and sequenced the core protein of fibroblast chondroitin sulfate proteoglycan. They designated it versican in recognition of its versatile modular structure. Versican belongs to the lectican protein family, with aggrecan (abundant in cartilage), brevican and neurocan (nervous system proteoglycans) as other members. Versican is also known as chondroitin sulfate proteoglycan core protein 2 or chondroitin sulfate proteoglycan 2 (CSPG2), and PG-M. Structure These proteoglycans share a homologous globular N-terminal, C-terminal, and glycosaminoglycan (GAG) binding regions. The N-terminal (G1) globular domain consists of Ig-like loop and two link modules, and has Hyaluronan (HA) binding pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate-GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser- Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |