|
Equivalent Temperature
In atmospheric science, equivalent temperature is the temperature of air in a parcel from which all the water vapor has been extracted by an adiabatic process. Air contains water vapor that has been evaporated into it from liquid sources (lakes, sea, etc...). The energy needed to do that has been taken from the air. Taking a volume of air at temperature and mixing ratio of , drying it by condensation will restore energy to the airmass. This will depend on the latent heat release as: T_e \approx T + \frac r where: * L_v : latent heat of evaporation (2400 kJ/kg at 25°C to 2600 kJ/kg at −40°C) * c_ : specific heat at constant pressure for air (≈ 1004 J/(kg·K)) Tables exist for exact values of the last two coefficients. See also * Wet-bulb temperature * Potential temperature * Atmospheric thermodynamics * Equivalent potential temperature Bibliography * M Robitzsch, ''Aequivalenttemperatur und Aequivalentthemometer'', Meteorologische Zeitschrift, 1928, pp. 313-315. * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmospheric Science
Atmospheric science is the study of the Atmosphere of Earth, Earth's atmosphere and its various inner-working physical processes. Meteorology includes atmospheric chemistry and atmospheric physics with a major focus on weather forecasting. Climatology is the study of atmospheric changes (both long and short-term) that define average climates and their change over time, due to both natural and anthropogenic climate change, climate variability. Aeronomy is the study of the upper layers of the atmosphere, where dissociation (chemistry), dissociation and ionization are important. Atmospheric science has been extended to the field of planetary science and the study of the atmospheres of the planets and natural satellites of the Solar System. Experimental instruments used in atmospheric science include satellites, rocketsondes, radiosondes, weather balloons, radars, and lasers. The term aerology (from Ancient Greek, Greek ἀήρ, ''aēr'', "air"; and -λογία, ''-logy, -logia'') is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature Of Air
Atmospheric temperature is a measure of temperature at different levels of the Earth's atmosphere. It is governed by many factors, including insolation, incoming solar radiation, humidity and altitude. When discussing surface air temperature, the annual atmospheric temperature range at any geographical location depends largely upon the type of biome, as measured by the Köppen climate classification Temperature versus altitude ] Temperature varies greatly at different heights relative to Earth's surface and this variation in temperature characterizes the four layers that exist in the atmosphere. These layers include the troposphere, stratosphere, mesosphere, and thermosphere. The troposphere is the lowest of the four layers, extending from the surface of the Earth to about into the atmosphere where the tropopause (the boundary between the troposphere stratosphere) is located. The width of the troposphere can vary depending on latitude, for example, the troposphere is thicker ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Air Parcel
In fluid dynamics, within the framework of continuum mechanics, a fluid parcel is a very small amount of fluid, identifiable throughout its dynamic history while moving with the fluid flow. As it moves, the mass of a fluid parcel remains constant, while—in a compressible flow—its volume may change. And its shape changes due to the distortion by the flow. In an incompressible flow the volume of the fluid parcel is also a constant ( isochoric flow). This mathematical concept is closely related to the description of fluid motion—its kinematics and dynamics—in a Lagrangian frame of reference. In this reference frame, fluid parcels are labelled and followed through space and time. But also in the Eulerian frame of reference the notion of fluid parcels can be advantageous, for instance in defining the material derivative, streamlines, streaklines, and pathlines; or for determining the Stokes drift. The fluid parcels, as used in continuum mechanics, are to be distinguished ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Vapor
(99.9839 °C) , - , Boiling point , , - , specific gas constant , 461.5 J/( kg·K) , - , Heat of vaporization , 2.27 MJ/kg , - , Heat capacity , 1.864 kJ/(kg·K) Water vapor, water vapour or aqueous vapor is the gaseous phase of water. It is one state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the atmosphere. Under typical atmospheric conditions, water vapor is continuously generated by evaporation and removed by condensation. It is less dense than most of the other constituents of air and triggers convection currents that can lead to clouds. Being a component of Earth's hydrosphere and hydrologic cycle, it is particularly abundant in Earth's atmosphere, where it acts as a greenhouse gas and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer. Thermometers are calibrated in various temperature scales that historically have relied on various reference points and thermometric substances for definition. The most common scales are the Celsius scale with the unit symbol °C (formerly called ''centigrade''), the Fahrenheit scale (°F), and the Kelvin scale (K), the latter being used predominantly for scientific purposes. The kelvin is one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic temperature scale. Experimentally, it can be approached very closely but not actually reached, as recognized in the third law of thermodynamics. It would be impossible to extract energy as heat from a body at that temperature. Temperature is important in all fields of natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mixing Ratio
In chemistry and physics, the dimensionless mixing ratio is the abundance of one component of a mixture relative to that of all other components. The term can refer either to mole ratio (see concentration) or mass ratio (see stoichiometry). In atmospheric chemistry and meteorology Mole ratio In atmospheric chemistry, mixing ratio usually refers to the mole ratio ''ri'', which is defined as the amount of a constituent ''ni'' divided by the total amount of all ''other'' constituents in a mixture: :r_i = \frac The mole ratio is also called amount ratio. If ''ni'' is much smaller than ''n''tot (which is the case for atmospheric trace constituents), the mole ratio is almost identical to the mole fraction. Mass ratio In meteorology, mixing ratio usually refers to the mass ratio of water \zeta, which is defined as the mass of water m_\mathrm divided by the mass of dry air (m_\mathrm-m_\mathrm) in a given air parcel: :\zeta = \frac The unit is typically given in \mathrm\,\mathrm^. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latent Heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition. Latent heat can be understood as energy in hidden form which is supplied or extracted to change the state of a substance without changing its temperature. Examples are latent heat of fusion and latent heat of vaporization involved in phase changes, i.e. a substance condensing or vaporizing at a specified temperature and pressure. The term was introduced around 1762 by Scottish chemist Joseph Black. It is derived from the Latin ''latere'' (''to lie hidden''). Black used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant. In contrast to latent heat, sensible heat is energy transferred as heat, with a resultant temperature change in a body. Usage The terms ″sensible heat″ and ″laten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Heat
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of of water by is , so the specific heat capacity of water is . Specific heat capacity often varies with temperature, and is different for each state of matter. Liquid water has one of the highest specific heat capacities among common substances, about at 20 °C; but that of ice, just below 0 °C, is only . The specific heat capacities of iron, granite, and hydrogen gas are about 449 J⋅kg−1⋅K−1, 790 J⋅kg−1⋅K−1, and 14300 J⋅kg−1⋅K−1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wet-bulb Temperature
The wet-bulb temperature (WBT) is the temperature read by a thermometer covered in water-soaked (water at ambient temperature) cloth (a wet-bulb thermometer) over which air is passed. At 100% relative humidity, the wet-bulb temperature is equal to the air temperature (dry-bulb temperature); at lower humidity the wet-bulb temperature is lower than dry-bulb temperature because of evaporative cooling. The wet-bulb temperature is defined as the temperature of a parcel of air cooled to saturation (100% relative humidity) by the evaporation of water into it, with the latent heat supplied by the parcel. A wet-bulb thermometer indicates a temperature close to the true (thermodynamic) wet-bulb temperature. The wet-bulb temperature is the lowest temperature that can be reached under current ambient conditions by the evaporation of water only. Even heat-adapted people cannot carry out normal outdoor activities past a wet-bulb temperature of , equivalent to a heat index of . The theoretic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
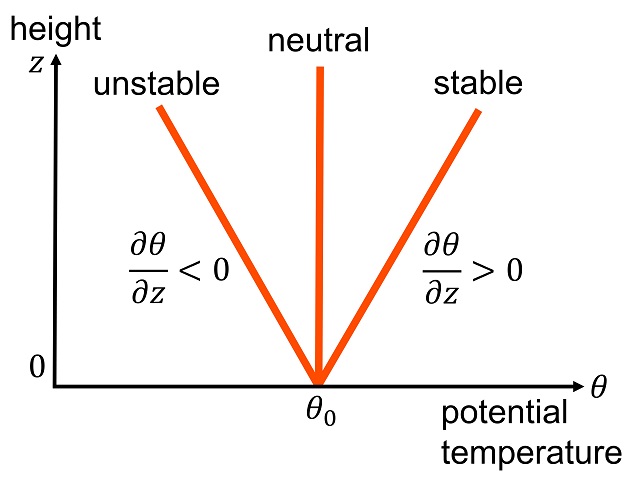
Potential Temperature
The potential temperature of a parcel of fluid at pressure P is the temperature that the parcel would attain if adiabatically brought to a standard reference pressure P_, usually . The potential temperature is denoted \theta and, for a gas well-approximated as ideal, is given by : \theta = T \left(\frac\right)^, where T is the current absolute temperature (in K) of the parcel, R is the gas constant of air, and c_p is the specific heat capacity at a constant pressure. R/c_p = 0.286 for air (meteorology). The reference point for potential temperature in the ocean is usually at the ocean's surface which has a water pressure of 0 dbar. The potential temperature in the ocean doesn't account for the varying heat capacities of seawater, therefore it is not a conservative measure of heat content. Graphical representation of potential temperature will always be less than the actual temperature line in a temperature vs depth graph. Contexts The concept of potential temperature applies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |