|
Epidermal Growth Factor Family
The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LDL Receptor
The low-density lipoprotein receptor (LDL-R) is a mosaic protein of 839 amino acids (after removal of 21-amino acid signal peptide) that mediates the endocytosis of cholesterol-rich low-density lipoprotein (LDL). It is a cell-surface receptor that recognizes apolipoprotein B100 (ApoB100), which is embedded in the outer phospholipid layer of very low-density lipoprotein (VLDL), their remnants—i.e. intermediate-density lipoprotein (IDL), and LDL particles. The receptor also recognizes apolipoprotein E (ApoE) which is found in chylomicron remnants and IDL. In humans, the LDL receptor protein is encoded by the gene on chromosome 19. It belongs to the low density lipoprotein receptor gene family. It is most significantly expressed in bronchial epithelial cells and adrenal gland and cortex tissue. Michael S. Brown and Joseph L. Goldstein were awarded the 1985 Nobel Prize in Physiology or Medicine for their identification of LDL-R and its relation to cholesterol metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gla Domain
Vitamin K-dependent carboxylation/gamma-carboxyglutamic (GLA) domain is a protein domain that contains post-translational modifications of many glutamate residues by vitamin K-dependent carboxylation to form γ-carboxyglutamate (Gla). Proteins with this domain are known informally as Gla proteins. The Gla residues are responsible for the high-affinity binding of calcium ions. The GLA domain binds calcium ions by chelating them between two carboxylic acid residues. These residues are part of a region that starts at the N-terminal extremity of the mature form of Gla proteins, and that ends with a conserved aromatic residue. This results in a conserved Gla-x(3)-Gla-x-Cys motif that is found in the middle of the domain, and which seems to be important for substrate recognition by the carboxylase. The 3D structures of several Gla domains have been solved. Calcium ions induce conformational changes in the Gla domain and are necessary for the Gla domain to fold properly. A common structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Ion
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor X
Factor X, also known by the eponym Stuart–Prower factor, is an enzyme () of the coagulation cascade. It is a serine endopeptidase (protease group S1, PA clan). Factor X is synthesized in the liver and requires vitamin K for its synthesis. Factor X is activated, by hydrolysis, into factor Xa by both factor IX (with its cofactor, factor VIII in a complex known as ''intrinsic tenase'') and factor VII with its cofactor, tissue factor (a complex known as ''extrinsic tenase''). It is therefore the first member of the ''final common pathway'' or ''thrombin pathway''. It acts by cleaving prothrombin in two places (an arg- thr and then an arg-ile bond), which yields the active thrombin. This process is optimized when factor Xa is complexed with activated co-factor V in the prothrombinase complex. Factor Xa is inactivated by protein Z-dependent protease inhibitor (ZPI), a serine protease inhibitor (serpin). The affinity of this protein for factor Xa is increased 1000-fold by the pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor IX
Factor IX (or Christmas factor) () is one of the serine proteases of the coagulation system; it belongs to peptidase family S1. Deficiency of this protein causes haemophilia B. It was discovered in 1952 after a young boy named Stephen Christmas was found to be lacking this exact factor, leading to haemophilia. Coagulation factor IX is on the World Health Organization's List of Essential Medicines. Physiology Factor IX is produced as a zymogen, an inactive precursor. It is processed to remove the signal peptide, glycosylated and then cleaved by factor XIa (of the contact pathway) or factor VIIa (of the tissue factor pathway) to produce a two-chain form, where the chains are linked by a disulfide bridge. When activated into factor IXa, in the presence of Ca2+, membrane phospholipids, and a Factor VIII cofactor, it hydrolyses one arginine-isoleucine bond in factor X to form factor Xa. Factor IX is inhibited by antithrombin. Factor IX expression increases with age in humans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Factor VII
Coagulation factor VII (, formerly known as proconvertin) is one of the proteins that causes blood to clot in the coagulation cascade, and in humans is coded for by the gene ''F7''. It is an enzyme of the serine protease class. Once bound to tissue factor released from damaged tissues, it is converted to factor VIIa (or ''blood-coagulation factor VIIa'', ''activated blood coagulation factor VII''), which in turn activates factor IX and factor X. Using genetic recombination a recombinant factor VIIa (eptacog alfa) (trade names include NovoSeven) has been approved by the FDA for the control of bleeding in hemophilia. It is sometimes used unlicensed in severe uncontrollable bleeding, although there have been safety concerns. A biosimilar form of recombinant activated factor VII (AryoSeven) is also available, but does not play any considerable role in the market. In April 2020, the US FDA approved a new rFVIIa product, eptacog beta (SEVENFACT), the first bypassing agent (BPA) appro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemophilia B
Haemophilia B, also spelled hemophilia B, is a blood clotting disorder causing easy bruising and bleeding due to an inherited mutation of the gene for factor IX, and resulting in a deficiency of factor IX. It is less common than factor VIII deficiency (haemophilia A). Haemophilia B was first recognized as a distinct disease entity in 1952. It is also known by the eponym ''Christmas disease'', named after Stephen Christmas, the first patient described with haemophilia B. In addition, the first report of its identification was published in the Christmas edition of the ''British Medical Journal''. Most individuals who have Hemophilia B and experience symptoms are men. The prevalence of Hemophilia B in the population is about one in 40,000; Hemophilia B represents about 15% of patients with hemophilia. Many women carriers of the disease have no symptoms. However, an estimated 10-25% of women carriers have mild symptoms; in rare cases, women may have moderate or severe symptoms. Si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marfan Syndrome
Marfan syndrome (MFS) is a multi-systemic genetic disorder that affects the connective tissue. Those with the condition tend to be tall and thin, with long arms, legs, fingers, and toes. They also typically have exceptionally flexible joints and abnormally curved spines. The most serious complications involve the heart and aorta, with an increased risk of mitral valve prolapse and aortic aneurysm. The lungs, eyes, bones, and the covering of the spinal cord are also commonly affected. The severity of the symptoms is variable. MFS is caused by a mutation in ''FBN1'', one of the genes that makes fibrillin, which results in abnormal connective tissue. It is an autosomal dominant disorder. In about 75% of cases, it is inherited from a parent with the condition, while in about 25% it is a new mutation. Diagnosis is often based on the Ghent criteria. There is no known cure for MFS. Many of those with the disorder have a normal life expectancy with proper treatment. Management of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidylserine
Phosphatidylserine (abbreviated Ptd-L-Ser or PS) is a phospholipid and is a component of the cell membrane. It plays a key role in cell cycle signaling, specifically in relation to apoptosis. It is a key pathway for viruses to enter cells via apoptotic mimicry. Its exposure on the outer surface of a membrane marks the cell for destruction via apoptosis. Structure Phosphatidylserine is a phospholipid—more specifically a glycerophospholipid—which consists of two fatty acids attached in ester linkage to the first and second carbon of glycerol and serine attached through a phosphodiester linkage to the third carbon of the glycerol. Phosphatidylserine sourced from plants differs in fatty acid composition from that sourced from animals. It is commonly found in the inner (cytoplasmic) leaflet of biological membranes. It is almost entirely found in the inner monolayer of the membrane with only less than 10% of it in the outer monolayer. Introduction Phosphatidylserine (PS) is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-linked Glycosylation
''N''-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom (the amide nitrogen of an asparagine (Asn) residue of a protein), in a process called ''N''-glycosylation, studied in biochemistry. This type of linkage is important for both the structure and function of many eukaryotic proteins. The ''N''-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of ''N''-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of ''N''-linked glycan. Energetics of bond formation There are two types of bonds involved in a glycoprotein: bonds between the saccharides residues in the glycan and the linkage between the glycan chain and the protein molecule. The sugar moieties are linked t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
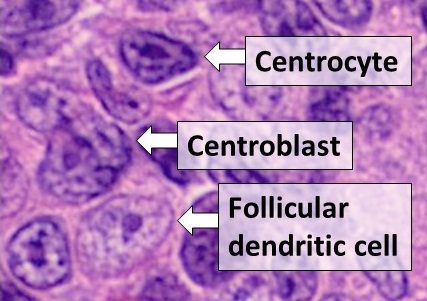
Dendritic Cells
Dendritic cells (DCs) are antigen-presenting cells (also known as ''accessory cells'') of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and the adaptive immune systems. Dendritic cells are present in those tissues that are in contact with the external environment, such as the skin (where there is a specialized dendritic cell type called the Langerhans cell) and the inner lining of the nose, lungs, stomach and intestines. They can also be found in an immature state in the blood. Once activated, they migrate to the lymph nodes where they interact with T cells and B cells to initiate and shape the adaptive immune response. At certain development stages they grow branched projections, the ''dendrites'' that give the cell its name (δένδρον or déndron being Greek for 'tree'). While similar in appearance, these are structures disti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |