|
Echinomycin
Echinomycin is a peptide antibiotic. It is a dimer of two peptides creating a cyclic structure. It contains a bicyclic aromatic chromophore that is attached to the dimerized cyclic peptide core and a thioacetal bridge. It intercalates into DNA at two specific sites, thereby blocking the binding of hypoxia inducible factor 1 alpha (HIF1alpha). Biosynthesis Echinomycin is a bis-intercalator peptide and is biosynthesized by a unique nonribosomal peptide synthetase (NRPS). Echinomycin is isolated from various bacteria such as ''Streptomyces'' ''lasalienis''. It belongs to a family of quinoxaline antibiotics. There is great interest in this group of compounds because they have very potent antibacterial, anticancer, and antiviral activities. The biosynthesis of echinomycin starts with molecule QC. L-tryptophan is the precursor for QC and its biosynthesis parallels the first stage of nikkomycin biosynthesis. After QC is biosynthesized, the adenylation domain-containing Ecm1 activa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioacetal Bridge Formation
In organosulfur chemistry, thioacetals are the sulfur (''thio-'') analogues of acetals (). There are two classes: the less-common monothioacetals, with the formula , and the dithioacetals, with the formula (symmetric dithioacetals) or (asymmetric dithioacetals). The symmetric dithioacetals are relatively common. They are prepared by condensation of thiols () or dithiols (two groups) with aldehydes (). These reactions proceed via the intermediacy of hemithioacetals (): #Thiol addition to give hemithioacetal: #::RSH + R'CH(O) -> R'CH(SR)OH #Thiol addition with loss of water to give dithioacetal: #::RSH + R'CH(OH)SR -> R'CH(SR)2 + H2O Such reactions typically employ either a Lewis acid or Brønsted acid as catalyst. Dithioacetals generated from aldehydes and either 1,2-ethanedithiol or 1,3-propanedithiol are especially common among this class of molecules for use in organic synthesis.P. Stütz And P. A. Stadler "3-alkylated And 3-acylated Indoles From A Common Precursor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl Carrier Protein
The acyl carrier protein (ACP) is a cofactor of both fatty acid and polyketide biosynthesis machinery. It is one of the most abundant proteins in cells of ''E. coli.'' In both cases, the growing chain is bound to the ACP via a thioester derived from the distal thiol of a 4'-phosphopantetheine moiety. Structure The ACPs are small negatively charged α-helical bundle proteins with a high degree of structural and amino acid similarity. The structures of a number of acyl carrier proteins have been solved using various NMR and crystallography techniques. The ACPs are related in structure and mechanism to the peptidyl carrier proteins (PCP) from nonribosomal peptide synthases. Biosynthesis Subsequent to the expression of the inactive ''apo'' ACP, the 4'-phosphopantetheine moiety is attached to a serine residue. This coupling is mediated by acyl carrier protein synthase (ACPS), a 4'-phosphopantetheinyl transferase. 4'-Phosphopantetheine is a prosthetic group of several acyl carrier pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structure Of Echinomycin Biosynthesis
A structure is an arrangement and organization of interrelated elements in a material object or system, or the object or system so organized. Material structures include man-made objects such as buildings and machines and natural objects such as biological organisms, minerals and chemicals. Abstract structures include data structures in computer science and musical form. Types of structure include a hierarchy (a cascade of one-to-many relationships), a network featuring many-to-many links, or a lattice featuring connections between components that are neighbors in space. Load-bearing Buildings, aircraft, skeletons, anthills, beaver dams, bridges and salt domes are all examples of load-bearing structures. The results of construction are divided into buildings and non-building structures, and make up the infrastructure of a human society. Built structures are broadly divided by their varying design approaches and standards, into categories including building structures, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
QXC Biosynthesis
Pilot licensing or certification refers to permits for operating aircraft. Flight crew licences are regulated by ICAO Annex 1 and issued by the civil aviation authority of each country. CAA’s have to establish that the holder has met a specific set of knowledge and experience before issuing the licence. The licence, with added ratings, allows a pilot to fly aircraft registered in the licence issuing state. The ICAO ''Annex 1 - Personnel Licensing'' acts as the international minimum standards for licensing, however states can deviate from these standards by notifying ICAO about the changes. This, for instance, is why there are certain differences regarding licensing between EASA in Europe and the FAA in the USA. Regulation by country In the United States, pilot certification is regulated by the Federal Aviation Administration (FAA), a branch of the U.S. Department of Transportation (DOT). A pilot is certified under the authority of Parts 61 and 141 of Title 14 of the Code of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
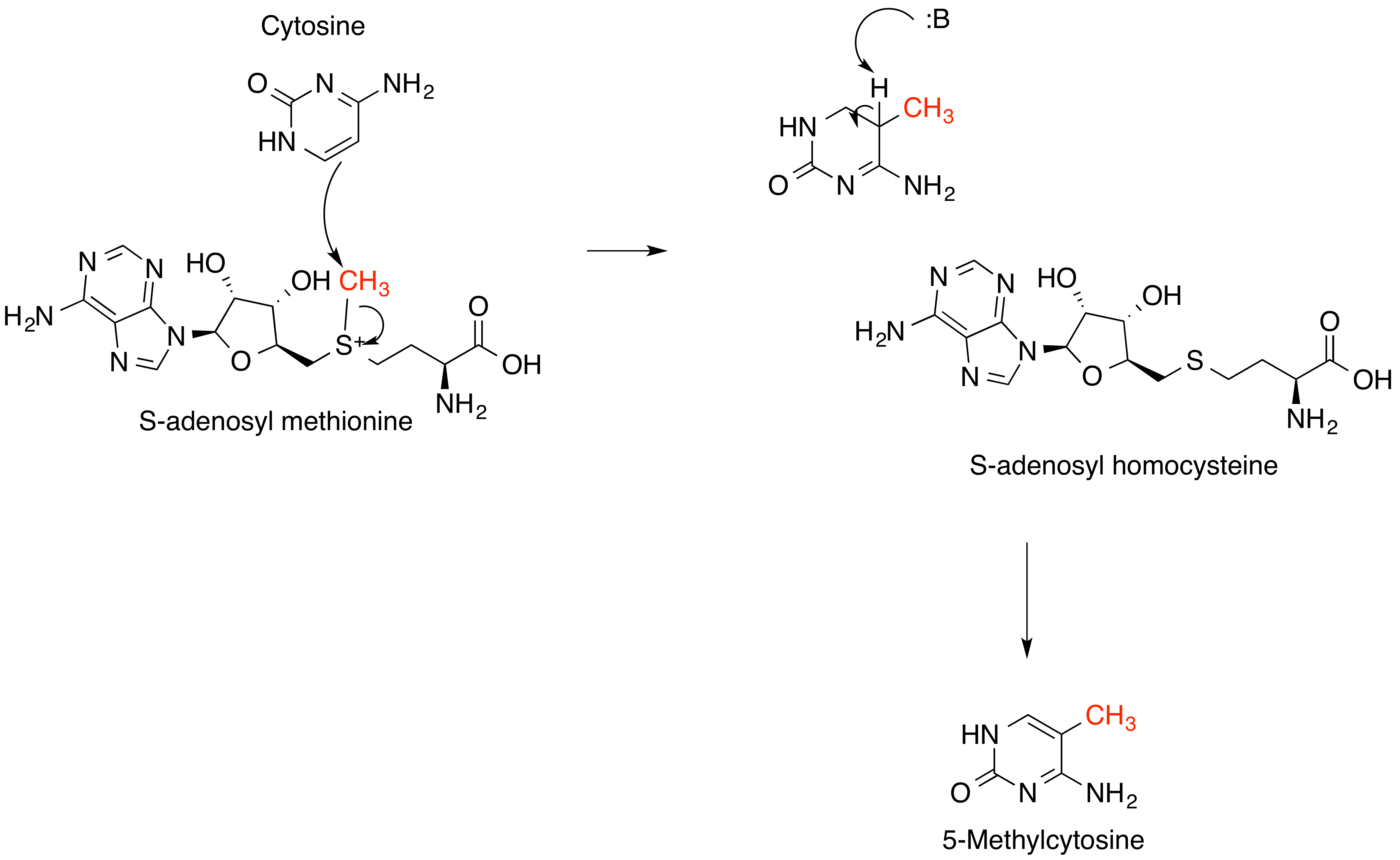
Methyltransferase
Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding ''S''-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S-adenosyl-L-methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioacetal
In organosulfur chemistry, thioacetals are the sulfur (''thio-'') analogues of acetals (). There are two classes: the less-common monothioacetals, with the formula , and the dithioacetals, with the formula (symmetric dithioacetals) or (asymmetric dithioacetals). The symmetric dithioacetals are relatively common. They are prepared by condensation of thiols () or dithiols (two groups) with aldehydes (). These reactions proceed via the intermediacy of hemithioacetals (): #Thiol addition to give hemithioacetal: #::RSH + R'CH(O) -> R'CH(SR)OH #Thiol addition with loss of water to give dithioacetal: #::RSH + R'CH(OH)SR -> R'CH(SR)2 + H2O Such reactions typically employ either a Lewis acid or Brønsted acid as catalyst. Dithioacetals generated from aldehydes and either 1,2-ethanedithiol or 1,3-propanedithiol are especially common among this class of molecules for use in organic synthesis.P. Stütz And P. A. Stadler "3-alkylated And 3-acylated Indoles From A Common Precursor: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. ''Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioesterase
Thioesterases are enzymes which belong to the esterase family. Esterases, in turn, are one type of the several hydrolases known. Thioesterases exhibit esterase activity (splitting of an ester into acid and Alcohol (chemistry), alcohol, in the presence of water) specifically at a thiol group. Thioesterases or thiolester hydrolases are identified as members of EC 3.1.2. Family The thioesterase activity is performed by members of the acyl-CoA thioesterase (ACOT) family. The regulatory role of ACOT in fatty acid metabolism depends on their substrate (biology), substrate specificity, tissue expression and subcellular localization. For example, deactivation of fatty acids at the ER may traffic fatty acids away from pathways associated with the ER membrane, such as glycerolipid biosynthesis. Two structurally different ACOT types lead to a similar enzymatic activity in vitro, dividing the family into type I and type II ACOTs. Type I ACOTs (ACOT1–6) contain the α/β-hydrolase domain, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenylation
Adenylylation, more commonly known as AMPylation, is a process in which an adenosine monophosphate (AMP) molecule is covalently attached to the amino acid side chain of a protein. This covalent addition of AMP to a hydroxyl side chain of the protein is a posttranslational modification. Adenylylation involves a phosphodiester bond between a hydroxyl group of the molecule undergoing adenylylation, and the phosphate group of the adenosine monophosphate nucleotide (i.e. adenylic acid). Enzymes that are capable of catalyzing this process are called AMPylators. The known amino acids to be targeted in the protein are tyrosine and threonine, and sometimes serine. When charges on a protein undergo a change, it affects the characteristics of the protein, normally by altering its shape via interactions of the amino acids which make up the protein. AMPylation can have various effects on the protein. These are properties of the protein like, stability, enzymatic activity, co-factor binding, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nikkomycin
Nikkomycins are a group of antifungal medications. They work by interfering with the building of the fungal cell wall which results in the fungal cell breaking open. They were discovered in 1976. The specific agent nikkomycin Z has weak activity against ''Aspergillus fumigatus'' which may be of benefit when used with other medications, such as caspofungin, ranconazole and amphotericin B, fluconazole or itraconazole. Nikkomycin Z also inhibits growth of ''Batrachochytrium dendrobatidis ''Batrachochytrium dendrobatidis'' ( ), also known as ''Bd'' or the amphibian chytrid fungus, is a fungus that causes the disease chytridiomycosis in amphibians. Since its discovery in 1998 by Lee Berger, the disease devastated amphibian popula ...'', a serious fungal pathogen linked to global amphibian declines, while lower concentrations of Nikkomycin Z enhanced natural amphibian antimicrobial skin peptide effectiveness ''in vitro''. Originally identified from '' Streptomyces tendae'', the nik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |