|
Drosocin
Drosocin is a 19-residue long antimicrobial peptide (AMP) of flies first isolated in the fruit fly ''Drosophila melanogaster'', and later shown to be conserved throughout the genus ''Drosophila''. Drosocin is regulated by the NF-κB Imd signalling pathway in the fly. The ''Drosocin'' gene encodes two peptides: its namesake Drosocin peptide and a second peptide called Buletin. Structure and function Drosocin is primarily active against Gram-negative bacteria. The peptide is proline-rich with proline-arginine repeats, as well a critical threonine residue. This threonine is ''O''-glycosylated, which is required for antimicrobial activity. This ''O''-glycosylation can be performed either by mono- or disaccharides, which have different activity spectra. Like the antimicrobial peptides pyrrhocoricin and abaecin, drosocin binds to bacterial DnaK, inhibiting cell machinery and replication. The action of these drosocin-like peptides is potentiated by the presence of pore-forming pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imd Pathway
The Imd pathway is a broadly-conserved NF-κB immune signalling pathway of insects and some arthropods that regulates a potent antibacterial defence response. The pathway is named after the discovery of a mutation causing severe immune deficiency (the gene was named ''"Imd"'' for "immune deficiency"). The Imd pathway was first discovered in 1995 using ''Drosophila'' fruit flies by Bruno Lemaitre and colleagues, who also later discovered that the ''Drosophila'' Toll gene regulated defence against Gram-positive bacteria and fungi. Together the Toll and Imd pathways have formed a paradigm of insect immune signalling; as of September 2, 2019, these two landmark discovery papers have been cited collectively over 5000 times since publication on Google Scholar. The Imd pathway responds to signals produced by Gram-negative bacteria. Peptidoglycan recognition proteins (PGRPs) sense DAP-type peptidoglycan, which activates the Imd signalling cascade. This culminates in the translocation of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Melanogaster
''Drosophila melanogaster'' is a species of fly (the taxonomic order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly" or "pomace fly". Starting with Charles W. Woodworth's 1901 proposal of the use of this species as a model organism, ''D. melanogaster'' continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and life history evolution. As of 2017, five Nobel Prizes have been awarded to drosophilists for their work using the insect. ''D. melanogaster'' is typically used in research owing to its rapid life cycle, relatively simple genetics with only four pairs of chromosomes, and large number of offspring per generation. It was originally an African species, with all non-African lineages having a common origin. Its geographic range includes all continents, including islands. ''D. melanogaster'' is a common pest in homes, restaurants, and othe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrhocoricin
Pyrrhocoricin is a 20-residue long antimicrobial peptide of the firebug ''Pyrrhocoris apterus''. Structure and function Pyrrhocoricin is primarily active against Gram-negative bacteria. The peptide is proline-rich with proline-arginine repeats, as well a critical threonine residue, which is required for activity through ''O''-glycosylation. Like the antimicrobial peptides drosocin and abaecin, pyrrhocoricin binds to the bacterial protein DnaK, inhibiting cell machinery and replication. Only the L-enantiomer of pyrrhocoricin is active against bacteria. The action of pyrrhocoricin-like peptides is potentiated by the presence of pore-forming peptides, which facilitates the entry of pyrrhocoricin-like peptides into the bacterial cell. Proline-rich peptides like Pyrrhocoricin can also bind to microbe ribosomes, preventing protein translation In molecular biology and genetics, translation is the process in which ribosomes in the cytoplasm or endoplasmic reticulum synthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Neotestacea
''Drosophila neotestacea'' is a member of the ''testacea'' species group of ''Drosophila''. Testacea species are specialist fruit flies that breed on the fruiting bodies of mushrooms. These flies will choose to breed on psychoactive mushrooms such as the Fly Agaric '' Amanita muscaria''. ''Drosophila neotestacea'' can be found in temperate regions of North America, ranging from the north eastern United States to western Canada. Immunity ''Drosophila neotestacea'' and other mushroom-breeding Drosophila have been studied extensively for their interactions with '' Howardula'' nematode parasites, particularly ''Howardula aoronymphium''. Unlike related species, ''D. neotestacea'' is sterilized by ''H. aoronymphium'' infection. The genetic basis of this susceptibility is unknown, and is nematode-dependent. For instance, a related ''Howardula'' species from Japan does not sterilize ''D. neotestacea'', even though the European and North American ''Howardula'' species do. Moreover, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enterobacter Cloacae
''Enterobacter cloacae'' is a clinically significant Gram-negative, facultatively-anaerobic, rod-shaped bacterium. Microbiology In microbiology labs, ''E. cloacae'' is frequently grown at 30 °C on nutrient agar or at 35 °C in tryptic soy broth. It is a rod-shaped, Gram-negative bacterium, is facultatively anaerobic, and bears peritrichous flagella. It is oxidase-negative and catalase-positive. Industrial use ''Enterobacter cloacae'' has been used in a bioreactor-based method for the biodegradation of explosives and in the biological control of plant diseases. ''Enterobacter cloacae'' strain MBB8 isolated from the Gulf of Mannar, India was reported to degrade poly vinyl alcohol (PVA). This was the first report of a PVA degrader from the Enterobacter genus. ''E. cloacae'' was also reported to produce exopolysaccharide (EPS) as high as 18.3g/L. GC-MS analysis of ''E. cloacae'' EPS showed the presence of glucose and mannose in the molar ratio of 1: 1.5e−2. Safe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Translation
In molecular biology and genetics, translation is the process in which ribosomes in the cytoplasm or endoplasmic reticulum synthesize proteins after the process of transcription of DNA to RNA in the cell's nucleus. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later folds into an active protein and performs its functions in the cell. The ribosome facilitates decoding by inducing the binding of complementary tRNA anticodon sequences to mRNA codons. The tRNAs carry specific amino acids that are chained together into a polypeptide as the mRNA passes through and is "read" by the ribosome. Translation proceeds in three phases: # Initiation: The ribosome assembles around the target mRNA. The first tRNA is attached at the start codon. # Elongation: The last tRNA validated by the small ribosomal subunit (''accommodati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribosome
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to form polypeptide chains. Ribosomes consist of two major components: the small and large ribosomal subunits. Each subunit consists of one or more ribosomal RNA (rRNA) molecules and many ribosomal proteins (RPs or r-proteins). The ribosomes and associated molecules are also known as the ''translational apparatus''. Overview The sequence of DNA that encodes the sequence of the amino acids in a protein is transcribed into a messenger RNA chain. Ribosomes bind to messenger RNAs and use their sequences for determining the correct sequence of amino acids to generate a given protein. Amino acids are selected and carried to the ribosome by transfer RNA (tRNA) molecules, which enter the ribosome and bind to the messenger RNA chain via an anti-c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can unde ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylated
Glycosylation is the reaction in which a carbohydrate (or 'glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Five class ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
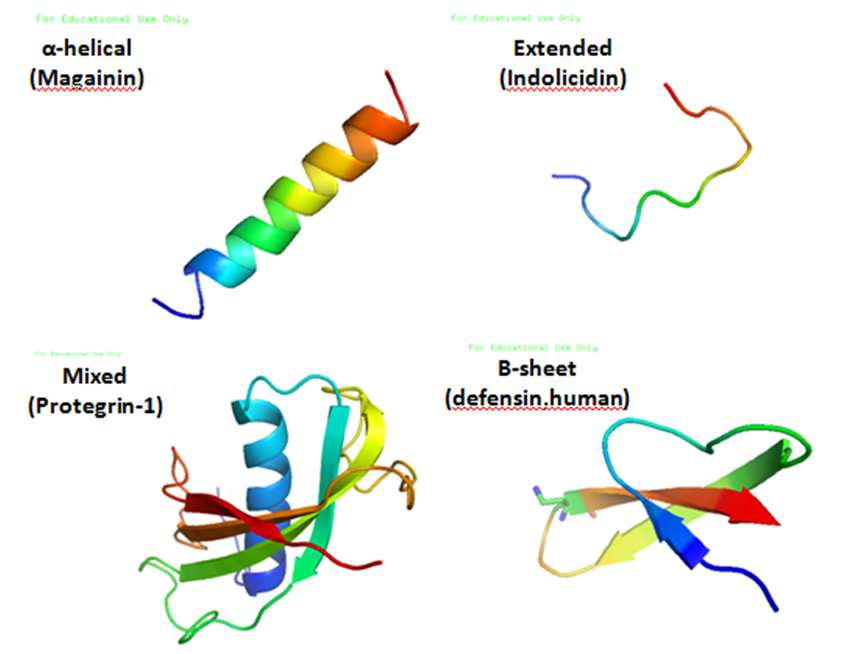
Antimicrobial Peptide
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are ge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |