|
Disulfur Monoxide
Disulfur monoxide or sulfur suboxide is an inorganic compound with formula S2O, one of the lower sulfur oxides. It is a colourless gas and condenses to give a roughly dark red coloured solid that is unstable at room temperature. occurs rarely in natural atmospheres, but can be made by a variety of laboratory procedures. For this reason, its spectroscopic signature is very well understood. Structure and spectrum Condensed solid S2O absorbs at (roughly indigo) and (roughly lime). These bands have been assigned to decomposition products S3 and S4. In the ultraviolet, S2O has absorption band systems in the ranges 250–340 nm and 190–240 nm. There are bands at 323.5 and 327.8 nm. The band in the 315–340 nm range is due to the transition. Gaseous disulfur monoxide does not absorb in the visible spectrum. The microwave spectrum of S2O has the following rotational parameters: ''A'' = 41915.44 MHz, ''B'' = 5059.07 MHz, and ''C''&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trisulfur
The molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. It comprises about 10% of vaporised sulfur at and . It has been observed at cryogenic temperatures as a solid. Under ordinary conditions it converts to cyclooctasulfur. :8 S3 → 3 S8 Structure and bonding In terms of structure and bonding and ozone () are similar. Both adopt bent structures and are diamagnetic. Although represented with S=S double bonds, the bonding situation is more complex. The S–S distances are equivalent and are , and with an angle at the central atom of . However, cyclic , where the sulfur atoms are arranged in an equilateral triangle with three single bonds (similar to cyclic ozone and cyclopropane), is calculated to be lower in energy than the bent structure experimentally observed. The name thiozone was invented by Hugo Erdmann in 1908 who hypothesized that comprises a large proportion of liquid sulfur. However its existen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of Copper extraction, copper mining and the precursor to many other copper-containing products and chemical compounds. Production It is produced on a large scale by pyrometallurgy, as one stage in extracting copper from its ores. The ores are treated with an aqueous mixture of ammonium carbonate, ammonia, and oxygen to give copper(I) and copper(II) ammine complexes, which are extracted from the solids. These complexes are decomposed with steam to give CuO. It can be formed by heating copper in air at around 300–800°C: : 2 Cu + O2 → 2 CuO For laboratory uses, pure copper(II) oxide is better prepared by heating copper(II) nitrate, copper(II) hydroxide, or basic copper(II) carbonate: : 2 Cu(NO3)2(s) → 2 CuO(s) + 4 NO2(g) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gases
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma). A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture, such as air, contains a variety of pure gases. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colourless gas invisible to the human observer. The gaseous state of matter occurs between the liquid and plasma states, the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases which are gaining increasing attention. High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dithiirane 1-oxides
In organic chemistry, episulfides are a class of organic compounds that contain a saturated, heterocyclic ring consisting of two carbon atoms and one sulfur atom. It is the sulfur analogue of an epoxide or aziridine. They are also known as thiiranes, olefin sulfides, thioalkylene oxides, and thiacyclopropanes. Episulfides are less common and generally less stable than epoxides. The most common derivative is ethylene sulfide (). Structure According to electron diffraction, the and distances in ethylene sulfide are respectively 1.473 and 1.811 Å. The and angles are respectively 66.0 and 48.0°. Preparation History A number of chemists in the early 1900s, including Staudinger and Pfenninger (1916), as well as Delepine (1920) studied episulfides.Sander, M. Thiiranes. Chem. Rev. 1966, 66(3), 297-339. I 1934 Dachlauer and Jackel devised a general synthesis of episulfides from epoxides using alkali thiocyanates and thiourea. Contemporary methods Following the lead of Da ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazoalkane
The diazo group is an organic moiety consisting of two linked nitrogen atoms ( azo) at the terminal position. Overall charge neutral organic compounds containing the diazo group bound to a carbon atom are called diazo compounds or diazoalkanes and are described by the general structural formula R2C=N+=N–. The simplest example of a diazo compound is diazomethane, CH2N2. Diazo compounds (R2C=N2) should not be confused with azo compounds of the type R-N=N-R or with diazonium compounds of the type R-N2+. Structure The electronic structure of diazo compounds is characterized by π electron density delocalized over the α-carbon and two nitrogen atoms, along with an orthogonal π system with electron density delocalized over only the terminal nitrogen atoms. Because all octet rule-satisfying resonance forms of diazo compounds have formal charges, they are members of a class of compounds known as 1,3-dipoles. Some of the most stable diazo compounds are α-diazo-β-diketones and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
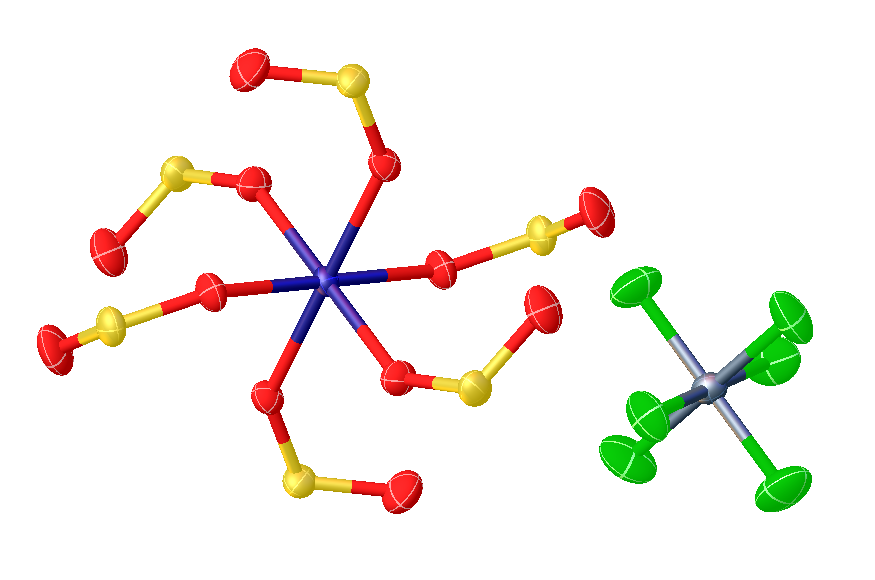
Transition Metal Sulfur Dioxide Complex
In organometallic chemistry, metal sulfur dioxide complexes are complexes that contain sulfur dioxide, , bonded to a transition metal. Such compounds are common but are mainly of theoretical interest. Historically, the study of these compounds has provided insights into the mechanisms of migratory insertion reactions. Bonding modes Sulfur dioxide forms complexes with many transition metals. Most numerous are complexes with metals i in oxidation state 0 or +1. In most cases SO2 binds in monodentate fashion, attaching to the metal through sulfur. Such complexes are further subdivided according to the planarity or pyramidalization at sulfur. The various bonding modes are: * η1-SO2, planar (meaning that the MSO2 subunit forms a plane). In such complexes, SO2 is classified as a 2e donor complemented by pi-back bonding into the empty pz orbital localized on sulfur. * η1-SO2, pyramidal (meaning that the MSO2 subunit is pyramidal at sulfur). In such complexes, SO2 is classi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hapticity
In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated (otherwise the κ-notation is used). In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity (not hapticity), and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands. History The need for additional nomenclature for organometallic compounds became apparent in the mid-1950s when Dunitz, Orgel, and Rich described the structure of the "sandwich complex" ferrocene by X-ray crystallography where an iron atom is ''"sandwiched"'' between two parallel cyclopent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can use d orbitals as valence orbitals to form chemical bonds. The lanthanide and actinide elements (the f-block) are called inner transition metals and are sometimes considered to be transition metals as well. Since they are metals, they are lustrous and have good electrical and thermal conductivity. Most (with the exception of group 11 and group 12) are hard and strong, and have high melting and boiling temperatures. They form compounds in any of two or more different oxidation states and bind to a variety of ligands to form coordination complexes that are often coloured. They form many useful alloys and are often employed as catalysts in elemental form or in compounds such as coordination complexes and oxides. Most are strongly paramag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incomplete Combustion
Combustion, or burning, is a high-temperature exothermic redox chemical reaction between a fuel (the reductant) and an oxidant, usually atmospheric oxygen, that produces oxidized, often gaseous products, in a mixture termed as smoke. Combustion does not always result in fire, because a flame is only visible when substances undergoing combustion vaporize, but when it does, a flame is a characteristic indicator of the reaction. While the activation energy must be overcome to initiate combustion (e.g., using a lit match to light a fire), the heat from a flame may provide enough energy to make the reaction self-sustaining. Combustion is often a complicated sequence of elementary radical reactions. Solid fuels, such as wood and coal, first undergo endothermic pyrolysis to produce gaseous fuels whose combustion then supplies the heat required to produce more of them. Combustion is often hot enough that incandescent light in the form of either glowing or a flame is produced. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pele (volcano)
Pele is an active volcano on the surface of Jupiter's moon Io. It is located on Io's trailing hemisphere at A large, tall volcanic plume has been observed at Pele by various spacecraft starting with ''Voyager 1'' in 1979, though it has not been persistent. The discovery of the Pele plume on March 8, 1979 confirmed the existence of active volcanism on Io. The plume is associated with a lava lake at the northern end of the mountain Danube Planum. Pele is also notable for a persistent, large red ring circling the volcano resulting from sulfurous fallout from the volcanic plume. Observations ''Voyager'' As ''Voyager 1'' approached the Jupiter system in March 1979, it acquired numerous images of the planet and its four largest satellites, including Io. One of the most distinctive features of these distant images of Io was a large, elliptical, footprint-shaped ring on the satellite's trailing hemisphere (the side facing away from the direction of motion in a synchronously-r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Io (moon)
Io (), or Jupiter I, is the innermost and third-largest of the four Galilean moons of the planet Jupiter. Slightly larger than Earth’s moon, Io is the fourth-largest moon in the Solar System, has the highest density of any moon, the strongest surface gravity of any moon, and the lowest amount of water (by atomic ratio) of any known astronomical object in the Solar System. It was discovered in 1610 by Galileo Galilei and was named after the mythological character Io, a priestess of Hera who became one of Zeus's lovers. With over 400 active volcanoes, Io is the most geologically active object in the Solar System. This extreme geologic activity is the result of tidal heating from friction generated within Io's interior as it is pulled between Jupiter and the other Galilean moons—Europa, Ganymede and Callisto. Several volcanoes produce plumes of sulfur and sulfur dioxide that climb as high as above the surface. Io's surface is also dotted with more than 100 mountains t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.png)

4-3D-balls.png)

.jpg)
