|
Dispase
Dispase is a protease which cleaves fibronectin, collagen IV, and to a lesser extent collagen I. It is found in some bacteria and can be isolated from culture filtrates of ''Bacillus polymyxa''. It can be extracted, purified, and used in research. It can be particularly useful to separate embryonic epithelia and mesenchyme. Dispase II is specific for the cleavage of leucine-phenylalanine bonds. Dispase is often used to digest adhering primary cells in culture, since this treatment turned out to be milder than trypsin digestion (Sinclair et al., 2013). A recent article also finds that dispase can digest serine-phenylalanine. Dispase intravitreal injection can be used in the modeling of proliferative vitreoretinopathy Proliferative vitreoretinopathy (PVR) is a disease that develops as a complication of rhegmatogenous retinal detachment. PVR occurs in about 8–10% of patients undergoing primary retinal detachment surgery and prevents the successful surgical re ... in different ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacillus Polymyxa
''Paenibacillus polymyxa'', also known as ''Bacillus polymyxa'', is a Gram-positive bacterium capable of fixing nitrogen. It is found in soil, plant tissues, marine sediments and hot springs. It may have a role in forest ecosystems and potential future applications as a biofertilizer and biocontrol agent in agriculture. Growth conditions ''P. polymyxa'' can be grown in the laboratory on trypticase soy agar medium. Applications Agricultural use ''P. polymyxa'' might have possible future applications as a soil inoculant in agriculture and horticulture. Biofilms of ''P. polymyxa'' growing on plant roots have been shown to produce exopolysaccharides which protect the plants from pathogens. The interactions between this bacterial species and plant roots also cause the root hairs to undergo physical changes. Antibiotics Some strains of ''P. polymyxa'' produce antibiotics including fusaricidin and polymyxins. ''P. polymyxa'' var. ''colistinus'' produces the antibiotic colistin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proliferative Vitreoretinopathy
Proliferative vitreoretinopathy (PVR) is a disease that develops as a complication of rhegmatogenous retinal detachment. PVR occurs in about 8–10% of patients undergoing primary retinal detachment surgery and prevents the successful surgical repair of rhegmatogenous retinal detachment. PVR can be treated with surgery to reattach the detached retina but the visual outcome of the surgery is very poor. A number of studies have explored various possible adjunctive agents for the prevention and treatment of PVR, such as methotrexate, although none have yet been licensed for clinical use. PVR was originally referred to as massive vitreous retraction and then as massive periretinal proliferation. The name Proliferative vitreo retinopathy was provided in 1989 by the Silicone Oil Study group. The name is derived from ''proliferation'' (by the retinal pigment epithelium, retinal pigment epithelial and glial cells) and ''vitreo retinopathy'' to include the tissues which are affected, namel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
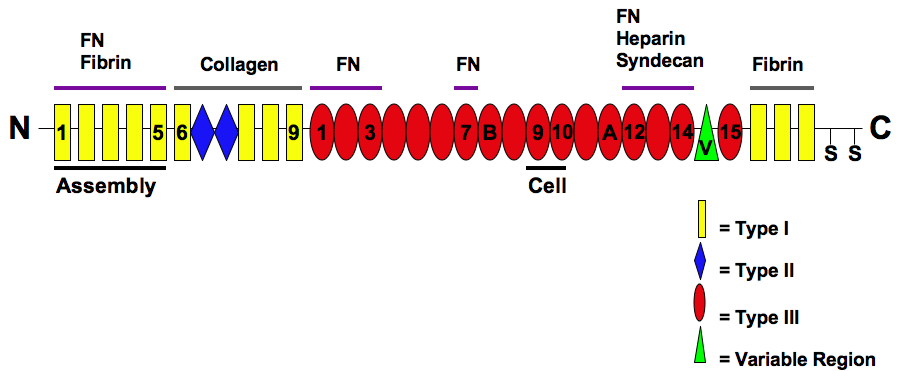
Fibronectin
Fibronectin is a high- molecular weight (~500-~600 kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans (e.g. syndecans). Fibronectin exists as a protein dimer, consisting of two nearly identical monomers linked by a pair of disulfide bonds. The fibronectin protein is produced from a single gene, but alternative splicing of its pre-mRNA leads to the creation of several isoforms. Two types of fibronectin are present in vertebrates: * soluble plasma fibronectin (formerly called "cold-insoluble globulin", or CIg) is a major protein component of blood plasma (300 μg/ml) and is produced in the liver by hepatocytes. * insoluble cellular fibronectin is a major component of the extracellular matrix. It is secreted by various cells, primarily fibroblasts, as a soluble protein dimer and is then ass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Depending upon the degree of mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes one to two percent of muscle tissue and accounts for 6% of the weight of the skeletal muscle tissue. The fibroblast is the most common cell that crea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic relationsh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epithelia
Epithelium or epithelial tissue is one of the four basic types of animal tissue, along with connective tissue, muscle tissue and nervous tissue. It is a thin, continuous, protective layer of compactly packed cells with a little intercellular matrix. Epithelial tissues line the outer surfaces of organs and blood vessels throughout the body, as well as the inner surfaces of cavities in many internal organs. An example is the epidermis, the outermost layer of the skin. There are three principal shapes of epithelial cell: squamous (scaly), columnar, and cuboidal. These can be arranged in a singular layer of cells as simple epithelium, either squamous, columnar, or cuboidal, or in layers of two or more cells deep as stratified (layered), or ''compound'', either squamous, columnar or cuboidal. In some tissues, a layer of columnar cells may appear to be stratified due to the placement of the nuclei. This sort of tissue is called pseudostratified. All glands are made up of epithelia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesenchyme
Mesenchyme () is a type of loosely organized animal embryonic connective tissue of undifferentiated cells that give rise to most tissues, such as skin, blood or bone. The interactions between mesenchyme and epithelium help to form nearly every organ in the developing embryo. Vertebrates Structure Mesenchyme is characterized morphologically by a prominent ground substance matrix containing a loose aggregate of reticular fibers and unspecialized mesenchymal stem cells. Mesenchymal cells can migrate easily (in contrast to epithelial cells, which lack mobility), are organized into closely adherent sheets, and are polarized in an apical-basal orientation. Development The mesenchyme originates from the mesoderm. From the mesoderm, the mesenchyme appears as an embryologically primitive "soup". This "soup" exists as a combination of the mesenchymal cells plus serous fluid plus the many different tissue proteins. Serous fluid is typically stocked with the many serous elements, such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Like valine and isoleucine, leucine is a branched-chain amino acid. The primary metabolic end products of leucine metabolism are acetyl-CoA and acetoacetate; consequently, it is one of the two exclusively ketogenic amino acids, with lysine being the other. It is the most import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin. It is encoded by the codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement for its analgesic and antidepressant effects. It is a direct precursor to the neuromodulator phenethylamine, a commonly used dietary supplement. As an essential amino acid, phenylalanine is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Function In the duodenum, trypsin catalyzes the hydrolysis of pept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |