|
Diisopropyl Azodicarboxylate
Diisopropyl azodicarboxylate (DIAD) is the diisopropyl ester of azodicarboxylic acid. It is used as a reagent in the production of many organic compounds. It is often used in the Mitsunobu reaction, where it serves as an oxidizer of triphenylphosphine to triphenylphosphine oxide. It has also been used to generate aza- Baylis-Hillman adducts with acrylates. It can also serve as a selective deprotectant of N-benzyl groups in the presence of other protecting groups. It is sometimes preferred to diethyl azodicarboxylate (DEAD) because it is more hindered, and thus less likely to form hydrazide Hydrazides in organic chemistry are a class of organic compounds with the formula RNHNH2 where R is acyl (R'CO-), sulfonyl (R'SO2-), or phosphoryl (R'2P(O)-). Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive infl ... byproducts. One notable use of this compound is in the synthesis of Bifenazate (Floramite®). References {{reflist Azo compounds Carboxyla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protecting Groups
A protecting group or protective group is introduced into a molecule by chemical modification of a functional group to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis. In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminium hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the ace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
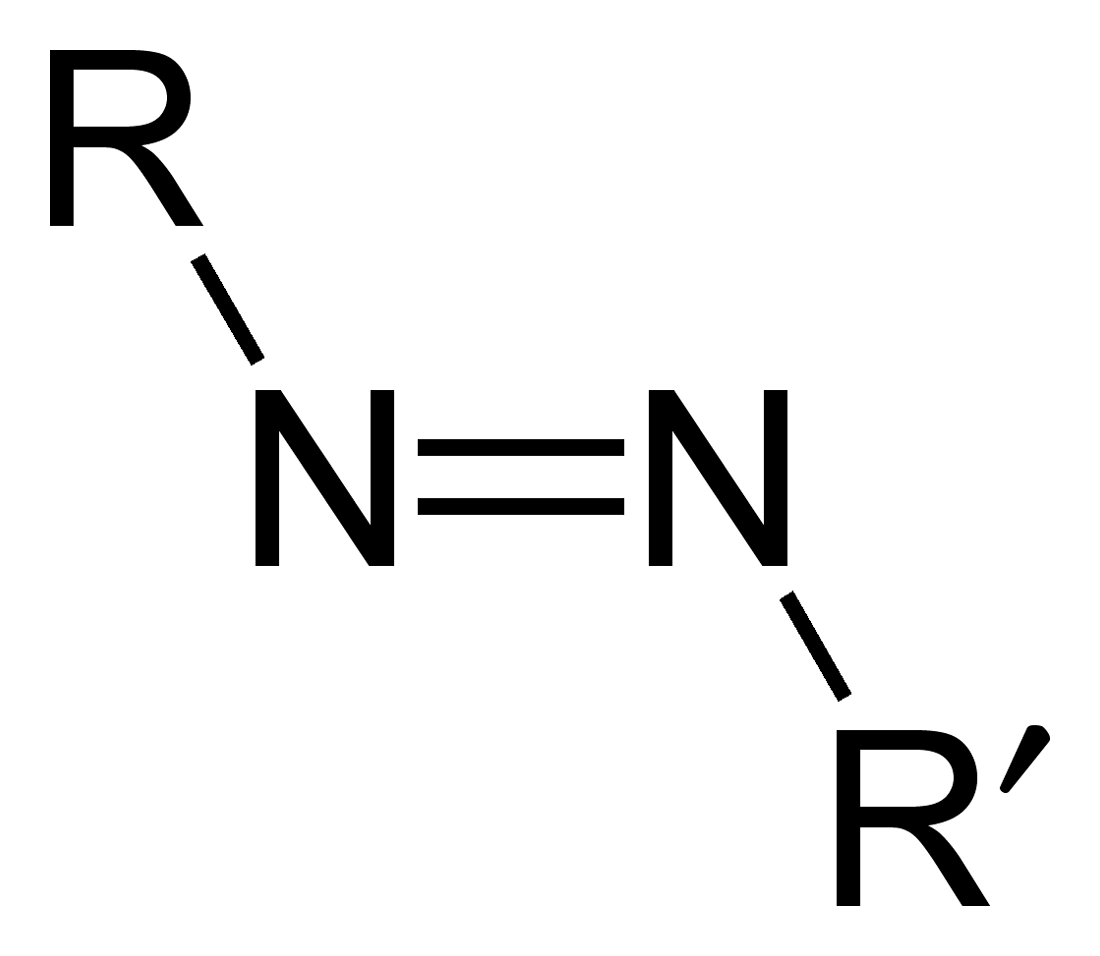
Azo Compounds
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazide
Hydrazides in organic chemistry are a class of organic compounds with the formula RNHNH2 where R is acyl (R'CO-), sulfonyl (R'SO2-), or phosphoryl (R'2P(O)-). Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive influence of the acyl, sulfonyl, or phosphoryl substituent. Sulfonyl hydrazides A common sulfonyl hydrazide is p-toluenesulfonyl hydrazide, a white air-stable solid. They are also widely used as organic reagents. Toluenesulfonyl hydrazide is used to generate toluenesulfonyl hydrazones. When derived from ketones, these hydrazones participate in the Shapiro reaction and the Eschenmoser–Tanabe fragmentation. 2,4,6-Triisopropylbenzenesulfonylhydrazide is a useful source of diimide. Acyl hydrazide Acylhydrazines are derivatives of carboxylic acids, although they are typically prepared by the reaction of esters with hydrazine: Use An applied example is a synthesis of sunitinib begins by mixing 5-fluoroisatin slowly into hydrazine hydrate. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Azodicarboxylate
Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2O2CN=NCO2CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles. DEAD is an aza- dienophile and an efficient dehydrogenating agent, converting alcohols to aldehydes, thiols to di sulfides and hydrazo groups to azo groups; it is also a good electron acceptor. While DEAD is used in numerous chemical reactions it is mostly known as a key component of the Mitsunobu reaction, a common strategy for the preparation of an amine, azide, ether, thioether, or ester from the corresponding alcohol. It is used in the synthesis of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthesis (journal)
''Synthesis'' is a scientific journal published from 1969 to the present day by Thieme Medical Publishers, Thieme. Its stated purpose is the "advancement of the science of synthetic chemistry". From August 2006, selected articles are offered free of charge. The impact factor of this journal is 2.867 (2018).Journal Citation Reports, 2018 References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group. Nomenclature In IUPAC nomenclature, the prefix benzyl refers to a substituent, for example benzyl chloride or benzyl benzoate. Benzyl is not to be confused with phenyl with the formula . The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring. For example, is referred to as a "benzylic" carbocation. The benzyl free radical has the formula . The benzyl cation or phenylcarbenium ion is the carbocation with formula ; the benzyl anion or phenylmethanide ion is the carbanion with the formula . None of these species can be formed in significant amounts in the solution phase under normal conditions, but they are useful referents for discussion of reaction mechanisms and may exist as reactive intermediates. Abbreviations The abbreviation "Bn" denotes benzyl. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron (journal)
''Tetrahedron'' is a weekly peer-reviewed scientific journal covering the field of organic chemistry. According to the ''Journal Citation Reports'', ''Tetrahedron'' has a 2020 impact factor of 2.457. ''Tetrahedron'' and Elsevier, its publisher, support an annual symposium. In 2010, complaints were raised over its high subscription cost. Notable papers , the Web of Science lists ten papers from ''Tetrahedron'' that have more than 1000 citations. The four articles that have been cited more than 2000 times are: * – cited 2228 times * – cited 2162 times * – cited 2124 times * – cited 2107 times See also * ''Tetrahedron Letters'' * ''Tetrahedron Computer Methodology'' * ''Polyhedron In geometry, a polyhedron (plural polyhedra or polyhedrons; ) is a three-dimensional shape with flat polygonal faces, straight edges and sharp corners or vertices. A convex polyhedron is the convex hull of finitely many points, not all on th ...'' (journal) Refere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = phenyl, C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallization, crystallizing of chemical compounds. Structure and properties Ph3PO is a tetrahedral molecule related to POCl3. The oxygen center is relatively basic. The rigidity of the backbone and the basicity of the oxygen center make this species a popular agent to crystallize otherwise difficult to crystallize molecules. This trick is applicable to molecules that have acidic hydrogen atoms, e.g. phenols. Up to now, several modifications of Ph3PO have been found: For example, a monoclinic form crystalizes in the space group ''P''21/''c'' with Z = 4 and a = 15.066(1) Å, b = 9.037(2) Å, c = 11.296(3) Å, and β = 98.47(1)°. The orthorhombic modif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



nickel(II)-from-xtal-3D-balls.png)