|
Distortion Free Energy Density
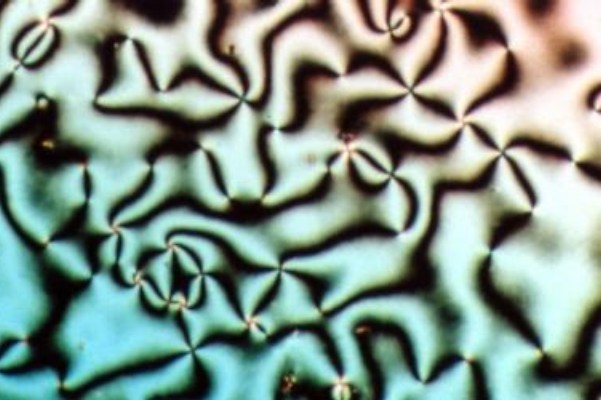
The distortion free energy density is a quantity that describes the increase in the free energy density of a liquid crystal caused by distortions from its uniformly aligned configuration. It also commonly goes by the name Frank free energy density named after Frederick Charles Frank. Nematic liquid crystal The distortion free energy density in a nematic liquid crystal is a measure of the increase in the Helmholtz free energy In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature (isothermal In thermodynamics, an isotherma ... per unit volume due to deviations in the orientational ordering away from a uniformly aligned nematic director configuration. The total free energy density for a nematic is therefore given by: :\mathcal_=\mathcal_+\mathcal_ , where \mathcal_ is the total free energy density of a liquid crystal, \mathcal_ is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystal
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal may flow like a liquid, but its molecules may be oriented in a crystal-like way. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. LC materials may not always be in a LC state of matter (just as water may be ice or water vapor). Liquid crystals can be divided into 3 main types: * thermotropic, *lyotropic, and * metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions as a function of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick Charles Frank
Sir Frederick Charles Frank, OBE, FRS (6 March 1911 – 5 April 1998) was a British theoretical physicist. He is best known for his work on crystal dislocations, including (with Thornton Read) the idea of the Frank–Read source of dislocations. He also proposed the cyclol reaction in the mid-1930s, and made many other contributions to solid-state physics, geophysics, and the theory of liquid crystals. Early life and education He was born in Durban, South Africa, although his parents returned to England soon afterwards. He was educated at Thetford Grammar School and Ipswich School and went on to study chemistry at Lincoln College, Oxford, gaining a doctorate at the university's Engineering Laboratory. Career Prior to World War II, he worked as a physicist in Berlin and as a colloid chemist in Cambridge. During World War II he joined the Chemical Defence Experimental Station at Porton Down, Wiltshire, but in 1940 was transferred to the Air Ministry's Assistant Directorate of I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helmholtz Free Energy
In thermodynamics, the Helmholtz free energy (or Helmholtz energy) is a thermodynamic potential that measures the useful work obtainable from a closed thermodynamic system at a constant temperature (isothermal In thermodynamics, an isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and ...). The change in the Helmholtz energy during a process is equal to the maximum amount of work that the system can perform in a thermodynamic process in which temperature is held constant. At constant temperature, the Helmholtz free energy is minimized at equilibrium. In contrast, the Gibbs free energy or free enthalpy is most commonly used as a measure of thermodynamic potential (especially in chemistry) when it is convenient for applications that occur at constant ''pressure''. For example, in explosives research Helmholtz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dyne
The dyne (symbol: dyn; ) is a derived unit of force specified in the centimetre–gram–second (CGS) system of units, a predecessor of the modern SI. History The name dyne was first proposed as a CGS unit of force in 1873 by a Committee of the British Association for the Advancement of Science. Definition The dyne is defined as "the force required to accelerate a mass of one gram at a rate of one centimetre per second squared". An equivalent definition of the dyne is "that force which, acting for one second, will produce a change of velocity of one centimetre per second in a mass of one gram". One dyne is equal to 10 micronewtons, 10−5 N or to 10 nsn (nano sthenes) in the old metre–tonne–second system of units. : 1 dyn = 1 g⋅cm/s2 = 10−5 kg⋅m/s2 = 10−5 N : 1 N = 1 kg⋅m/s2 = 105 g⋅cm/s2 = 105 dyn Use The dyne per centimetre is a unit traditionally used to measure surface tension. For example, the surface tension of distilled water is 71.99 dyn/c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |