|
Dissipation
In thermodynamics, dissipation is the result of an irreversible process that affects a thermodynamic system. In a dissipative process, energy ( internal, bulk flow kinetic, or system potential) transforms from an initial form to a final form, where the capacity of the final form to do thermodynamic work is less than that of the initial form. For example, transfer of energy as heat is dissipative because it is a transfer of energy other than by thermodynamic work or by transfer of matter, and spreads previously concentrated energy. Following the second law of thermodynamics, in conduction and radiation from one body to another, the entropy varies with temperature (reduces the capacity of the combination of the two bodies to do work), but never decreases in an isolated system. In mechanical engineering, dissipation is the irreversible conversion of mechanical energy into thermal energy with an associated increase in entropy. Processes with defined local temperature produce ent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irreversible Process
In thermodynamics, an irreversible process is a thermodynamic processes, process that cannot be undone. All complex natural processes are irreversible, although a phase transition at the coexistence temperature (e.g. melting of ice cubes in water) is well approximated as reversible. A change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. A system that undergoes an irreversible process may still be capable of returning to its initial state. Because entropy is a state function, the change in entropy of the system is the same whether the process is reversible or irreversible. However, the impossibility occurs in restoring the Environment (systems), environment to its own initial conditions. An irreversible process increases the total entropy of the system and its surroundings. The second law of thermodynamics can be used to dete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
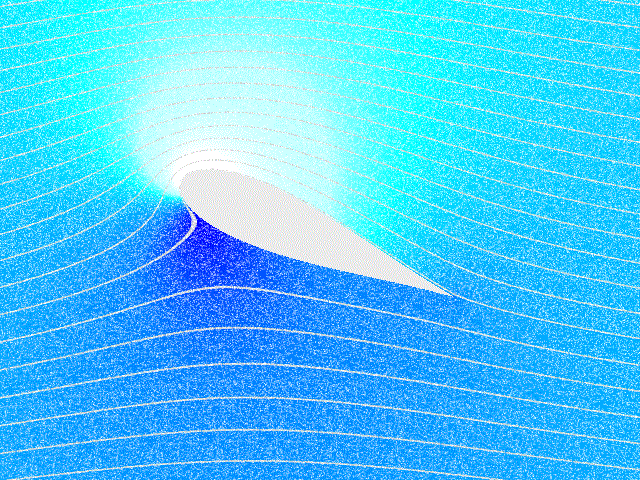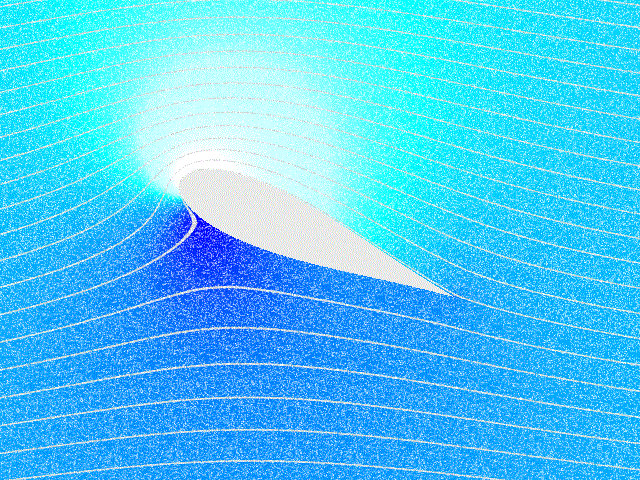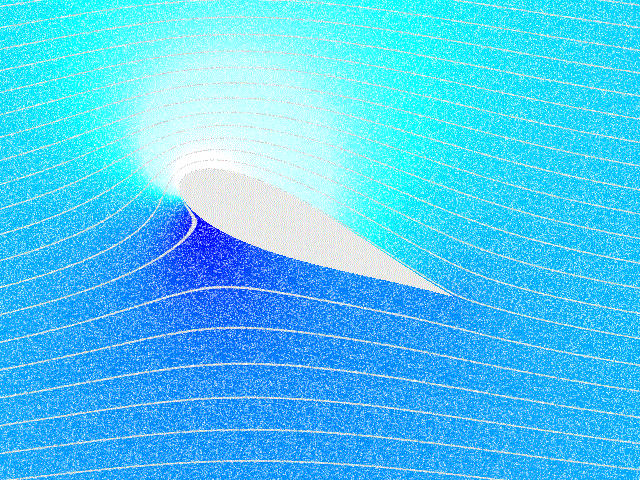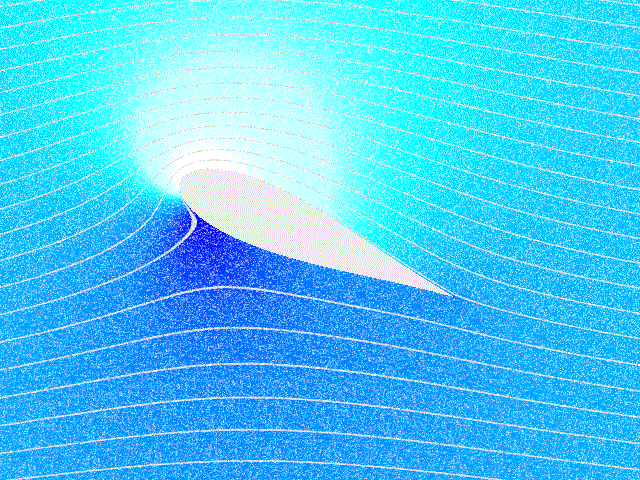
Fluid Flow
In physics, physical chemistry and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids – liquids and gases. It has several subdisciplines, including (the study of air and other gases in motion) and (the study of water and other liquids in motion). Fluid dynamics has a wide range of applications, including calculating forces and moment (physics), moments on aircraft, determining the mass flow rate of petroleum through pipeline transport, pipelines, weather forecasting, predicting weather patterns, understanding nebulae in interstellar space, understanding large scale Geophysical fluid dynamics, geophysical flows involving oceans/atmosphere and Nuclear weapon design, modelling fission weapon detonation. Fluid dynamics offers a systematic structure—which underlies these practical disciplines—that embraces empirical and semi-empirical laws derived from flow measurement and used to solve practical problems. The solution to a fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Resistance And Conductance
The electrical resistance of an object is a measure of its opposition to the flow of electric current. Its Multiplicative inverse, reciprocal quantity is , measuring the ease with which an electric current passes. Electrical resistance shares some conceptual parallels with mechanical friction. The International System of Units, SI unit of electrical resistance is the ohm (), while electrical conductance is measured in siemens (unit), siemens (S) (formerly called the 'mho' and then represented by ). The resistance of an object depends in large part on the material it is made of. Objects made of electrical insulators like rubber tend to have very high resistance and low conductance, while objects made of electrical conductors like metals tend to have very low resistance and high conductance. This relationship is quantified by electrical resistivity and conductivity, resistivity or conductivity. The nature of a material is not the only factor in resistance and conductance, howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy
Entropy is a scientific concept, most commonly associated with states of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. It has found far-ranging applications in chemistry and physics, in biological systems and their relation to life, in cosmology, economics, sociology, weather science, climate change and information systems including the transmission of information in telecommunication. Entropy is central to the second law of thermodynamics, which states that the entropy of an isolated system left to spontaneous evolution cannot decrease with time. As a result, isolated systems evolve toward thermodynamic equilibrium, where the entropy is highest. A consequence of the second law of thermodynamics is that certain processes are irreversible. The thermodynami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamiltonian Mechanics
In physics, Hamiltonian mechanics is a reformulation of Lagrangian mechanics that emerged in 1833. Introduced by Sir William Rowan Hamilton, Hamiltonian mechanics replaces (generalized) velocities \dot q^i used in Lagrangian mechanics with (generalized) ''momenta''. Both theories provide interpretations of classical mechanics and describe the same physical phenomena. Hamiltonian mechanics has a close relationship with geometry (notably, symplectic geometry and Poisson structures) and serves as a Hamilton–Jacobi equation, link between classical and quantum mechanics. Overview Phase space coordinates (''p'', ''q'') and Hamiltonian ''H'' Let (M, \mathcal L) be a Lagrangian mechanics, mechanical system with configuration space (physics), configuration space M and smooth Lagrangian_mechanics#Lagrangian, Lagrangian \mathcal L. Select a standard coordinate system (\boldsymbol,\boldsymbol) on M. The quantities \textstyle p_i(\boldsymbol,\boldsymbol,t) ~\stackrel~ / are called ''m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
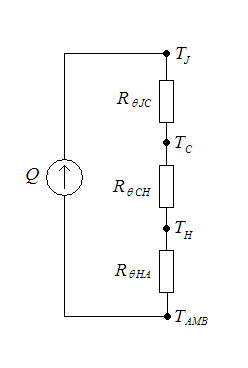
Thermal Resistance
In heat transfer, thermal engineering, and thermodynamics, thermal conductance and thermal resistance are fundamental concepts that describe the ability of materials or systems to conduct heat and the opposition they offer to the heat current. The ability to manipulate these properties allows engineers to control temperature gradient, prevent thermal shock, and maximize the efficiency of thermal systems. Furthermore, these principles find applications in a multitude of fields, including materials science, mechanical engineering, electronics, and energy management. Knowledge of these principles is crucial in various scientific, engineering, and everyday applications, from designing efficient temperature control, thermal insulation, and thermal management in industrial processes to optimizing the performance of electronic devices. Thermal conductance (''G'') measures the ability of a material or system to conduct heat. It provides insights into the ease with which heat can pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Production
Entropy production (or generation) is the amount of entropy which is produced during heat process to evaluate the efficiency of the process. Short history Entropy is produced in irreversible processes. The importance of avoiding irreversible processes (hence reducing the entropy production) was recognized as early as 1824 by Carnot. In 1865 Rudolf Clausius expanded his previous work from 1854 on the concept of "unkompensierte Verwandlungen" (uncompensated transformations), which, in our modern nomenclature, would be called the entropy production. In the same article in which he introduced the name entropy, Clausius gives the expression for the entropy production for a cyclical process in a closed system, which he denotes by ''N'', in equation (71) which reads :N=S-S_0-\int\frac. Here ''S'' is the entropy in the final state and ''S0'' the entropy in the initial state; ''S0-S'' is the entropy difference for the backwards part of the process. The integral is to be taken from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge carriers, which may be one of several types of particles, depending on the Electrical conductor, conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or Electron hole, holes. In an Electrolyte#Electrochemistry, electrolyte the charge carriers are ions, while in Plasma (physics), plasma, an Ionization, ionized gas, they are ions and electrons. In the International System of Units (SI), electric current is expressed in Unit of measurement, units of ampere (sometimes called an "amp", symbol A), which is equivalent to one coulomb per second. The ampere is an SI base unit and electric current is a ISQ base quantity, base quantity in the International System of Qua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on Universal (metaphysics), universal empirical observation concerning heat and Energy transformation, energy interconversions. A simple statement of the law is that heat always flows spontaneously from hotter to colder regions of matter (or 'downhill' in terms of the temperature gradient). Another statement is: "Not all heat can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics establishes the concept of entropy as a physical property of a thermodynamic system. It predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. For example, the first law allows the process of a cup falling off a table and breaking on the floor, as well as allowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Numerical Diffusion
Numerical diffusion is a difficulty with computer simulations of continua (such as fluids) wherein the simulated medium exhibits a higher diffusivity than the true medium. This phenomenon can be particularly egregious when the system should not be diffusive at all, for example an ideal fluid acquiring some spurious viscosity in a numerical model. Explanation In Eulerian simulations, time and space are divided into a discrete grid and the continuous differential equations of motion (such as the Navier–Stokes equation) are discretized into finite-difference equations. The discrete equations are in general more diffusive than the original differential equations, so that the simulated system behaves differently than the intended physical system. The amount and character of the difference depends on the system being simulated and the type of discretization that is used. Most fluid dynamics or magnetohydrodynamic simulations seek to reduce numerical diffusion to the minim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and light. Energy is a Conservation law, conserved quantity—the law of conservation of energy states that energy can be Energy transformation, converted in form, but not created or destroyed. The unit of measurement for energy in the International System of Units (SI) is the joule (J). Forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a Classical field theory, field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, the internal energy contained within a thermodynamic system, and rest energy associated with an object's rest mass. These are not mutual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy (energy Dispersal)
In thermodynamics, the interpretation of entropy as a measure of energy dispersal has been exercised against the background of the traditional view, introduced by Ludwig Boltzmann, of entropy as a quantitative measure of disorder. The energy dispersal approach avoids the ambiguous term ' disorder'. An early advocate of the energy dispersal conception was Edward A. Guggenheim in 1949, using the word 'spread'.Dugdale, J.S. (1996). ''Entropy and its Physical Meaning'', Taylor & Francis, London, , Dugdale cites only Guggenheim, on page 101.Guggenheim, E.A. (1949), Statistical basis of thermodynamics, ''Research: A Journal of Science and its Applications'', 2, Butterworths, London, pp. 450–454. In this alternative approach, entropy is a measure of energy ''dispersal'' or ''spread'' at a specific temperature. Changes in entropy can be quantitatively related to the distribution or the spreading out of the energy of a thermodynamic system, divided by its temperature. Some educators pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |