|
Diaminopimelate Decarboxylase
The enzyme diaminopimelate decarboxylase () catalyzes the cleavage of carbon-carbon bonds in meso 2,6 diaminoheptanedioate to produce CO2 and L-lysine, the essential amino acid. It employs the cofactor pyridoxal phosphate, also known as PLP, which participates in numerous enzymatic transamination, decarboxylation and deamination reactions. This enzyme belongs to the family of lyases, specifically the carboxy-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is ''meso''-2,6-diaminoheptanedioate carboxy-lyase (L-lysine-forming).DAP-decarboxylase catalyzes the final step in the meso-diaminopimelate/lysine biosynthetic pathway. Lysine is used for protein synthesis and used in the peptidoglycan layer of Gram-positive bacteria cell walls. This enzyme is not found in humans, but the ortholog is ornithine decarboxylase. Structure DAPDC is a PLP-dependent enzyme belonging to the alanine racemase family. This enzyme is generally dimeric with each mono ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-sandwich
Beta-sandwich, β-sandwich domains consisting of 80 to 350 amino acids occur commonly in proteins. They are characterized by two opposing antiparallel beta sheets (β-sheets). The number of strands found in such domains may differ from one protein to another. β-sandwich domains are subdivided in a variety of different folds. The immunoglobulin-type fold found in antibodies (Ig-fold) consists of a sandwich arrangement of 7 and 9 antiparallel β-strands arranged in two β-sheets with a Greek-key topology. The Greek-key topology is also found in Human Transthyretin. The jelly-roll topology is found in carbohydrate binding proteins such as concanavalin A and various lectins, in the collagen binding domain of ''Staphylococcus aureus'' Adhesin and in modules that bind fibronectin as found in Tenascin (Third Fibronectin Type III Repeat). The L-type lectin domain is a variation of the jelly roll fold. The C2 domain A C2 domain is a protein structural domain involved in targeting pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline Epoxidation," Zimmerman, H. E.; Singer, L.; Thyagarajan, B. S. J. Am. Chem. Soc., 1959, 81, 108-116.Eliel, E., "Stereochemistry of Carbon Compound", McGraw-Hill, 1962 pp 434-436 In contrast, stereoselectivity is the property of a reactant mixture where a non-stereospecific mechanism allows for the formation of multiple products, but where one (or a subset) of the products is favored by factors, such as steric access, that are independent of the mechanism. A stereospecific mechanism ''specifies'' the stereochemical outcome of a given reactant, whereas a stereoselective reaction ''selects'' products from those made available by the same, non-specific mechanism acting on a g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarboxylic Acid
In organic chemistry, a dicarboxylic acid is an organic compound containing two carboxyl groups (). The general molecular formula for dicarboxylic acids can be written as , where R can be aliphatic or aromatic. In general, dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acids. Dicarboxylic acids are used in the preparation of copolymers such as polyamides and polyesters. The most widely used dicarboxylic acid in the industry is adipic acid, which is a precursor in the production of nylon. Other examples of dicarboxylic acids include aspartic acid and glutamic acid, two amino acids in the human body. The name can be abbreviated to diacid. Linear saturated dicarboxylic acids The general formula is .Boy Cornils, Peter Lappe "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry 2014, Wiley-VCH, Weinheim. The PubChem links gives access to more information on the compounds, including other names, ids, toxicity and safety ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamic Acid
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC. D-Aspartate is one of two D-amino acids commonly found in mammals. .html" ;"title="/sup>">/sup> In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
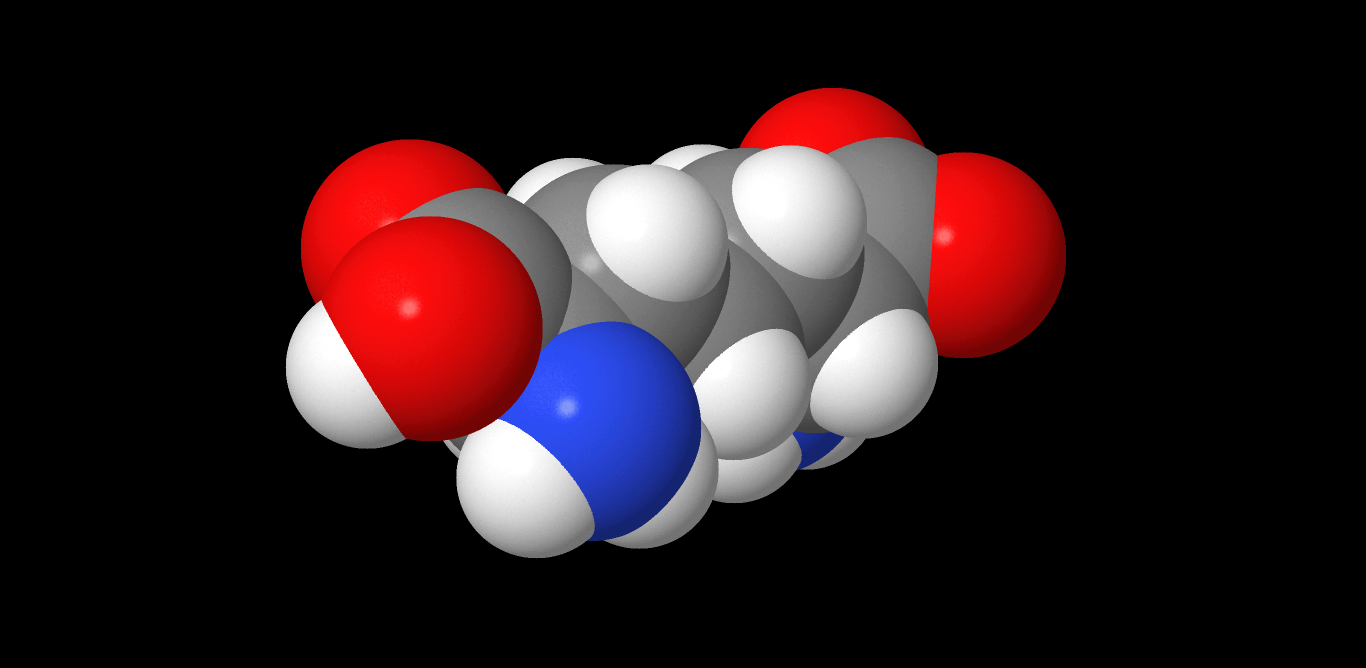
Diaminopimelic Acid
Diaminopimelic acid (DAP) is an amino acid, representing an epsilon-carboxy derivative of lysine. DAP is a characteristic of certain cell walls of some bacteria. DAP is often found in the peptide linkages of NAM-NAG chains that make up the cell wall of gram-negative bacteria. When provided, they exhibit normal growth. When in deficiency, they still grow but with the inability to make new cell wall peptidoglycan. This is also the attachment point for Braun's lipoprotein. See also * Aspartate-semialdehyde dehydrogenase, an enzyme involved in DAP synthesis * Peptidoglycan Peptidoglycan or murein is a unique large macromolecule, a polysaccharide, consisting of sugars and amino acids that forms a mesh-like peptidoglycan layer outside the plasma membrane, the rigid cell wall (murein sacculus) characteristic of most ba ... * Pimelic acid Images References Amino acids Dicarboxylic acids Non-proteinogenic amino acids {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DAP To Lysine
DAP or Dap may refer to: Science * DAP (gene), human gene that encodes death-associated proteins, which mediate programmed cell death * Diamidophosphate, phosphorylating compound * Diaminopimelic acid, amino acid derivative of lysine * Diaminopyridine, drug for the treatment of rare muscular diseases * Diaminopyrimidine, a class of organic chemicals * Diammonium phosphate, chemical used as a fertilizer and flame retardant * Dog appeasing pheromone, synthetic analogue of a canine hormone * Dose area product, a measurement of radiation exposure Technology * DAP (software), a statistical analysis program * Debug Access Port, for ARM processors * Digital Adoption Platform, a marketing term for software from WalkMe * Digital Archive Project, a collaboration for publishing non-mainstream TV programmes * Digital Audio Player, a class of electronic devices that play digital audio * Direct-Action Penetrator, the gunship version of the Sikorsky UH-60 Black Hawk helicopter * Directory Acc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol. In biochemistry, it similarly refers to a biomolecule formed of four units, that are the same (homotetramer), i.e. as in Concanavalin A or different (heterotetramer), i.e. as in hemoglobin. Hemoglobin has 4 similar sub-units while immunoglobulins have 2 very different sub-units. The different sub-units may have each their own activity, such as binding biotin in avidin tetramers, or have a common biological property, such as the allosteric binding of oxygen in hemoglobin. See also * Cluster chemistry; atomic and molecular clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycobacterium Tuberculosis
''Mycobacterium tuberculosis'' (M. tb) is a species of pathogenic bacteria in the family Mycobacteriaceae and the causative agent of tuberculosis. First discovered in 1882 by Robert Koch, ''M. tuberculosis'' has an unusual, waxy coating on its cell surface primarily due to the presence of mycolic acid. This coating makes the cells impervious to Gram staining, and as a result, ''M. tuberculosis'' can appear weakly Gram-positive. Acid-fastness, Acid-fast stains such as Ziehl–Neelsen stain, Ziehl–Neelsen, or Fluorescence, fluorescent stains such as Auramine O, auramine are used instead to identify ''M. tuberculosis'' with a microscope. The physiology of ''M. tuberculosis'' is highly aerobic organism, aerobic and requires high levels of oxygen. Primarily a pathogen of the mammalian respiratory system, it infects the lungs. The most frequently used diagnostic methods for tuberculosis are the Mantoux test, tuberculin skin test, Acid-Fast Stain, acid-fast stain, Microbiological cultu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |