|
Dialysate
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing. Dialysis is a common laboratory technique that operates on the same principle as medical dialysis. In the context of life science research, the most common application of dialysis is for the removal of unwanted small molecules such as salts, reducing agents, or dyes from larger macromolecules such as proteins, DNA, or polysaccharides. Dialysis is also commonly used for buffer exchange and drug binding studies. The concept of dialysis was introduced in 1861 by the Scottish chemist Thomas Graham. He used this technique to separate sucrose (small molecule) and gum Arabic solutes (large molecule) in aqueous solution. He called the diffusible solutes crystalloids and those that would not pass the membrane colloids. From this concept dialysis can be defined as a spontaneous separation process of suspen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kidney Dialysis
Kidney dialysis (from Greek , , 'dissolution'; from , , 'through', and , , 'loosening or splitting') is the process of removing excess water, solutes, and toxins from the blood in people whose kidneys can no longer perform these functions naturally. This is referred to as renal replacement therapy. The first successful dialysis was performed in 1943. Dialysis may need to be initiated when there is a sudden rapid loss of kidney function, known as acute kidney injury (previously called acute renal failure), or when a gradual decline in kidney function, chronic kidney disease, reaches stage 5. Stage 5 chronic renal failure is reached when the glomerular filtration rate is 10–15% of normal, creatinine clearance is less than 10 mL per minute and uremia is present. Dialysis is used as a temporary measure in either acute kidney injury or in those awaiting kidney transplant and as a permanent measure in those for whom a transplant is not indicated or not possible.Pendse S, Singh A, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition. The concept of diffusion is widely used in many fields, including physics (particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection. A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature with the change in another variable, usually distance. A change in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
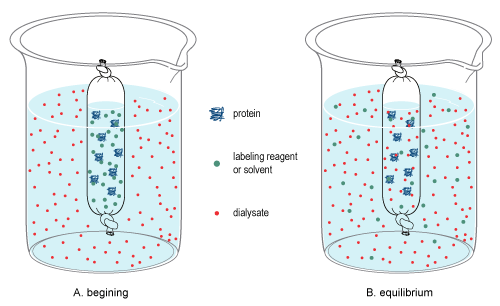
Dialysis Figure
Dialysis may refer to: *Dialysis (chemistry), a process of separating molecules in solution **Electrodialysis, used to transport salt ions from one solution to another through an ion-exchange membrane under the influence of an applied electric potential * Kidney dialysis is the process of removing water, solutes and toxins from the blood of individuals with compromised kidney function, primary types of which are: ** Hemodialysis ** Peritoneal dialysis ** Hemofiltration *Liver dialysis A liver support system or diachysis is a type of therapeutic device to assist in performing the functions of the liver. Such systems focus either on removing the accumulating toxins (liver dialysis), or providing additional replacement of the met ..., a detoxification treatment for liver failure * ''Dialysis'' (fly), a genus of insects in the family Xylophagidae {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation-exchange Membrane
An ion-exchange membrane is a semi-permeable membrane that transports certain dissolved ions, while blocking other ions or neutral molecules. Ion-exchange membranes are therefore electrically conductive. They are often used in desalination and chemical recovery applications, moving ions from one solution to another with little passage of water. Important examples of ion-exchange membranes include the proton-exchange membranes, that transport cations, and the anion exchange membranes used in certain alkaline fuel cells to transport anions. Structure and composition An ion-exchange membrane is generally made of organic or inorganic polymer with charged (ionic) side groups, such as ion-exchange resins. Anion-exchange membranes contain fixed cationic groups with predominantly mobile anions; because anions are the majority species, most of the conductivity is due to anion transport. The reverse holds for cation-exchange membranes. The so-called heterogeneous ion-exchange ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscose Process
Rayon is a semi-synthetic fiber, made from natural sources of regenerated cellulose, such as wood and related agricultural products. It has the same molecular structure as cellulose. It is also called viscose. Many types and grades of viscose fibers and films exist. Some imitate the feel and texture of natural fibers such as silk, wool, cotton, and linen. The types that resemble silk are often called artificial silk. The fibre is used to make textiles for clothing and other purposes. Rayon production involves solubilizing cellulose to allow turning the fibers into required form. Three common ways to solubilize are the cuprammonium process, not in use today, using ammoniacal solutions of copper salts; the viscose process, the most common today, using alkali and carbon sulfide; and the Lyocell process, using amine oxide. The last avoids the neurotoxic carbon sulfide of the viscose process but is also more expensive. Rayon and its variants Rayon is produced by dissolving ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemicellulose
A hemicellulose (also known as polyose) is one of a number of heteropolymer, heteropolymers (matrix polysaccharides), such as arabinoxylans, present along with cellulose in almost all embryophyte, terrestrial plant cell walls.Scheller HV, Ulvskov Hemicelluloses.// Annu Rev Plant Biol. 2010;61:263-89. doi: 10.1146/annurev-arplant-042809-112315. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or Base (chemistry), base as well as a myriad of Cellulase, hemicellulase enzymes. Composition Diverse kinds of hemicelluloses are known. Important examples include xylan, glucuronoxylan, arabinoxylan, glucomannan, and xyloglucan. Hemicelluloses are polysaccharides often associated with cellulose, but with distinct compositions and structures. Whereas cellulose is derived exclusively from glucose, hemicelluloses are composed of diverse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dialysis Tubing
Dialysis tubing, also known as ''Visking tubing'', is an artificial semi-permeable membrane tubing90% of a protein having a molecular mass of at least 10 kDa. Pore sizes typically range from ~10–100 Angstroms for 1K to 50K MWCO membranes. It is important to note that the MWCO of a membrane is not a sharply defined value. Molecules with mass near the MWCO of the membrane will diffuse across the membrane slower than molecules significantly smaller than the MWCO. In order for a molecule to rapidly diffuse across a membrane, it typically needs to be at least 20–50 times smaller than the membranes MWCO rating. Therefore, it is not practical to try separating a 30kDa protein from a 10kDa protein using dialysis across a 20K rated dialysis membrane. Dialysis tubing for laboratory use is typically made of a film of regenerated cellulose or cellulose ester. However; dialysis membranes made of polysulfone, polyethersulfone (PES), etched polycarbonate, or collagen are also extensively use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight Cut-off
Molecular weight cut-off or MWCO refers to the lowest molecular weight solute (in daltons) in which 90% of the solute is retained by the membrane, or the molecular weight of the molecule (e.g. globular protein) that is 90% retained by the membrane. This definition is not however standardized, and MWCOs can also be defined as the molecular weight at which 80% of the analytes (or solutes) are prohibited from membrane diffusion. Commercially available microdialysis probes typically have molecular weight cutoffs that range from 1,000 to 300,000 Da, and larger thresholds of filtration are measured in µm. Microdialysis may also be used to separate nanoparticle A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...s from the solutions in which they were formed. In such a separation, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight Cut-off
Molecular weight cut-off or MWCO refers to the lowest molecular weight solute (in daltons) in which 90% of the solute is retained by the membrane, or the molecular weight of the molecule (e.g. globular protein) that is 90% retained by the membrane. This definition is not however standardized, and MWCOs can also be defined as the molecular weight at which 80% of the analytes (or solutes) are prohibited from membrane diffusion. Commercially available microdialysis probes typically have molecular weight cutoffs that range from 1,000 to 300,000 Da, and larger thresholds of filtration are measured in µm. Microdialysis may also be used to separate nanoparticle A nanoparticle or ultrafine particle is usually defined as a particle of matter that is between 1 and 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 10 ...s from the solutions in which they were formed. In such a separation, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity". Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, William Nicholson and Anthony Carlisle sought to further Volt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_in_2002.png)


