|
Dehydroalanine
Dehydroalanine (Cα,β-didehydroalanine, α,β-di-dehydroalanine, 2-aminoacrylate, or 2,3-didehydroalanine) is a dehydroamino acid. It does not exist in its free form, but it occurs naturally as a residue found in peptides of microbial origin. As an amino acid residue, it is unusual because it has an unsaturated backbone. Structure and reactivity Like most primary enamines, dehydroalanine is unstable. Dehydroalanine hydrolyze to pyruvate. ''N''-Acylated derivatives of dehydroalanine, such as peptides and related compounds, are stable. For example, methyl 2-acetamidoacrylate is the N-acetylated derivative of the ester. As a residue in a peptide, it is generated by a post translational modification. The required precursors are serine or cysteine residues, which undergo enzyme-mediated loss of water and hydrogen sulfide, respectively. Most amino acid residues are unreactive toward nucleophiles, but those containing dehydroalanine or some other dehydroamino acids are exceptions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dehydroamino Acid
In biochemistry, a dehydroamino acid is an amino acids, usually with a C=C double bond in its side chain. Dehydroamino acids are not coded by DNA, but arise via post-transcriptional modification. Examples A common dehydroamino acid is dehydroalanine, which otherwise exists only as a residue in proteins and peptides. The dehydroalanine residue is obtained dehydration of serine-containing protein/peptide (alternatively, removal of H2S from cysteine). Another example is dehydrobutyrine, derived from dehydration of threonine. Generally, amino acid residues are unreactive toward nucleophiles, but the dehydroamino acids are exceptions to this pattern. For example, dehydroalanine adds cysteine and lysine to form covalent crosslinks. An unusual dehydroamino acid is dehydroglycine (DHG) because it does not contain a carbon-carbon double bond. Instead it is the imine of glyoxalic acid. It arises by the radical-induced degradation of tyrosine. N-Acyl derivatives Dehydroamino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcystin
Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria, commonly known as blue-green algae. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthases. Cyanobacteria can produce microcystins in large quantities during algal blooms which then pose a major threat to drinking and irrigation water supplies, and the environment at large. Characteristics Microcystins—or cyanoginosins—are a class of toxins produced by certain freshwater cyanobacteria; primarily ''Microcystis aeruginosa'' but also other ''Microcystis'', as well as members of the ''Planktothrix'', ''Anabaena'', ''Oscillatoria'' and ''Nostoc'' genera. Over 250 different microcystins have been discovered so far, of which microcystin-LR is the most common. Chemically they are cyclic heptapeptides produced through nonribosomal peptide synthase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine Ammonia-lyase
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and ''trans''-cinnamic acid.: :L-phenylalanine = ''trans''-cinnamate + NH3 Phenylalanine ammonia lyase (PAL) is the first and committed step in the phenyl propanoid pathway and is therefore involved in the biosynthesis of the polyphenol compounds such as flavonoids, phenylpropanoids, and lignin in plants. Phenylalanine ammonia lyase is found widely in plants, as well as some bacteria, yeast, and fungi, with isoenzymes existing within many different species. It has a molecular mass in the range of 270–330 kDa. The activity of PAL is induced dramatically in response to various stimuli such as tissue wounding, pathogenic attack, light, low temperatures, and hormones. PAL has recently been studied for possible therapeutic benefits in humans afflicted with phenylketonuria. It has also been used in the generation of L-phenylalanine as precursor of the sweetener asparta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthionine
Lanthionine is a nonproteinogenic amino acid with the chemical formula (HOOC-CH(NH2)-CH2-S-CH2-CH(NH2)-COOH). It is typically formed by a cysteine residue and a dehydrated serine residue. Despite its name, lanthionine does not contain the element lanthanum. Background In 1941, lanthionine was first isolated by treating wool with sodium carbonate. It was found to be a sulfur-containing amino acid; accordingly it was given the name lanthionine ool (Latin: ''Lana''), sulfur (Greek: ''theîon'') Lanthionine was first synthesized by alkylation of cysteine with β- chloroalanine. Lanthionines are found widely in nature. They have been isolated from human hair, lactalbumin, and feathers. Lanthionines have also been found in bacterial cell walls and are the components of a group of gene-encoded peptide antibiotics called lantibiotics, which includes nisin (a food preservative), subtilin, epidermin (effective against '' Staphylococcus'' and ''Streptococcus''), and ancovenin (an enzym ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
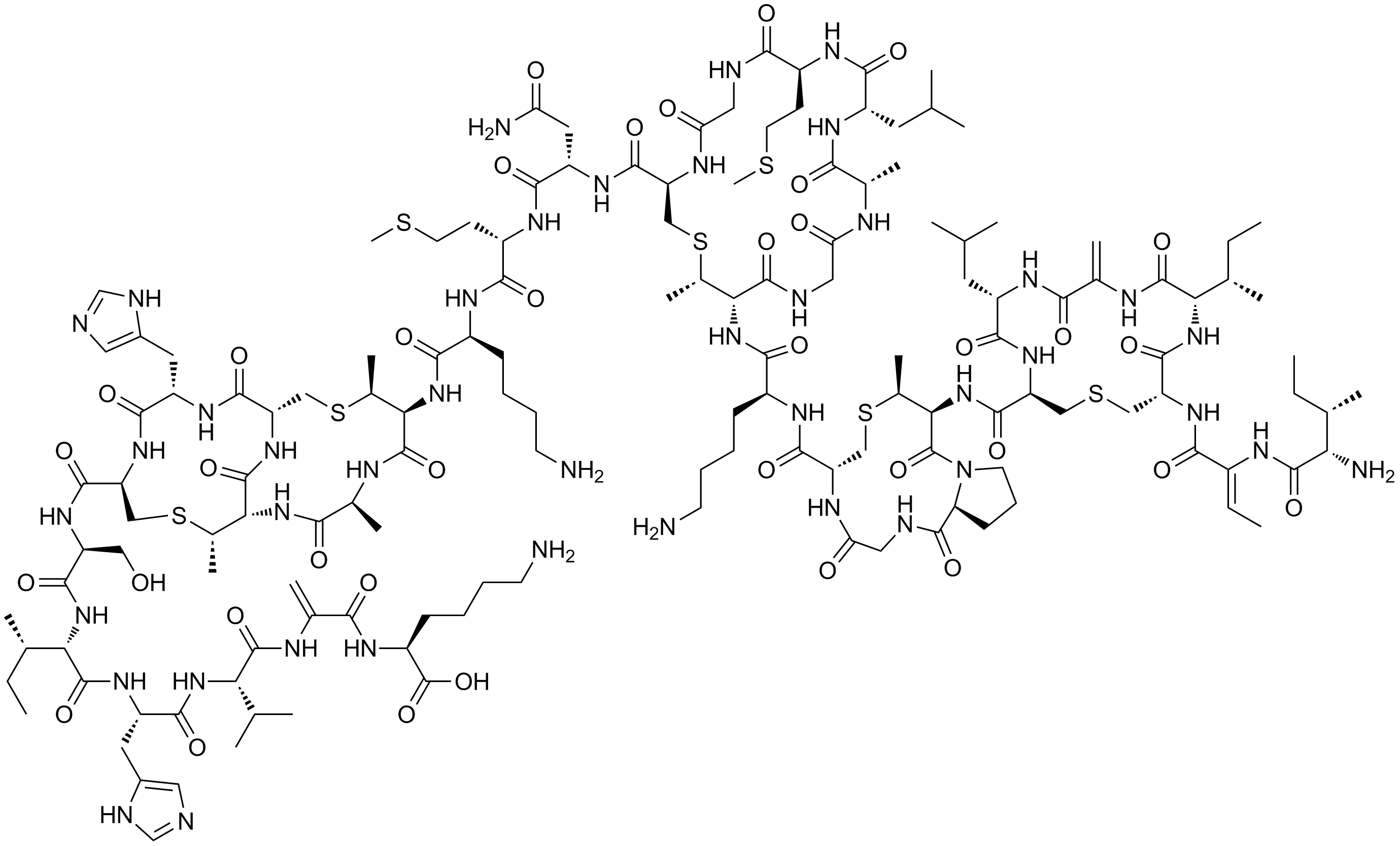
Nisin
Nisin is a polycyclic antibacterial peptide produced by the bacterium ''Lactococcus lactis'' that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), didehydroalanine (Dha), and didehydroaminobutyric acid (Dhb). These unusual amino acids are introduced by posttranslational modification of the precursor peptide. In these reactions a ribosomally synthesized 57-mer is converted to the final peptide. The unsaturated amino acids originate from serine and threonine, and the enzyme-catalysed addition of cysteine residues to the didehydro amino acids result in the multiple (5) thioether bridges. Subtilin and epidermin are related to nisin. All are members of a class of molecules known as lantibiotics. In the food industry, nisin is obtained from the culturing of ''L. lactis'' on natural substrates, such as milk or dextrose, and it is not chemically synthesized. It was originally isolated in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
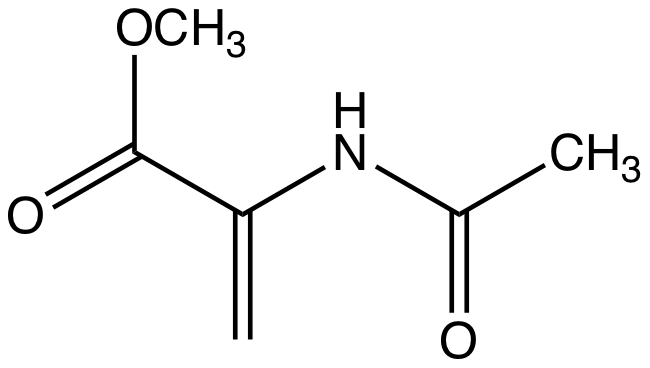
Methyl 2-acetamidoacrylate
Methyl 2-acetamidoacrylate is the organic compound with the formula CH2=C(NHC(O)CH3)CO2CH3. It is the methyl ester of an ''N''-acetylacrylic acid, which in turn is a derivative of the unstable compound dehydroalanine. Acetylation of the amine in the latter compound prevents tautomerization. It is a white solid. The compound can be prepared from methyl 2-acetamidopropionate (CH3CH(NHC(O)CH3)CO2CH3), i.e. the methyl ester of N-acetylalanine. Methyl 2-acetamidoacrylate undergoes Michael reaction In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...s, e.g. by thiolates. References {{Reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lantibiotics
Lantibiotics are a class of polycyclic peptide antibiotics that contain the characteristic thioether amino acids lanthionine or methyllanthionine, as well as the unsaturated amino acids dehydroalanine, and 2-aminoisobutyric acid. They belong to ribosomally synthesized and post-translationally modified peptides. Lanthionine is composed of two alanine residues that are crosslinked on their β-carbon atoms by a thioether (monosulfide) linkage. Lantibiotics are produced by a large number of Gram-positive bacteria such as ''Streptococcus'' and '' Streptomyces'' to attack other Gram-positive bacteria, and as such, they are considered a member of the bacteriocins. Bacteriocins are classified according to their extent of posttranslational modification. The lantibiotics are a class of more extensively modified bacteriocins, also called Class I bacteriocins. (Bacteriocins for which disulfide bonds are the only modification to the peptide are Class II bacteriocins.) Lantibiotics are w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nisin
Nisin is a polycyclic antibacterial peptide produced by the bacterium ''Lactococcus lactis'' that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), didehydroalanine (Dha), and didehydroaminobutyric acid (Dhb). These unusual amino acids are introduced by posttranslational modification of the precursor peptide. In these reactions a ribosomally synthesized 57-mer is converted to the final peptide. The unsaturated amino acids originate from serine and threonine, and the enzyme-catalysed addition of cysteine residues to the didehydro amino acids result in the multiple (5) thioether bridges. Subtilin and epidermin are related to nisin. All are members of a class of molecules known as lantibiotics. In the food industry, nisin is obtained from the culturing of ''L. lactis'' on natural substrates, such as milk or dextrose, and it is not chemically synthesized. It was originally isolated in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine Ammonia-lyase
Histidine ammonia-lyase (EC 4.3.1.3, histidase, histidinase) is an enzyme that in humans is encoded by the ''HAL'' gene. It converts histidine into ammonia and urocanic acid. Its systematic name is L-histidine ammonia-lyase (urocanate-forming). Function Histidine ammonia-lyase is a cytosolic enzyme catalyzing the first reaction in histidine catabolism, the nonoxidative deamination of L-histidine to ''trans''-urocanic acid. The reaction is catalyzed by 3,5-dihydro-5-methyldiene-4''H''-imidazol-4-one, an electrophilic co-factor which is formed autocatalytically by cyclization of the protein backbone of the enzyme. Pathology Mutations in the gene for histidase are associated with histidinemia and urocanic aciduria Urocanic aciduria is an autosomal recessive metabolic disorder caused by a deficiency of the enzyme urocanase. It is a secondary disorder of histidine metabolism.''Disorders of histidine metabolism.''http://www.ommbid.com/OMMBID/the_online_metabol .... References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. History The first known racemic mixture was racemic acid, which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid. He manually separated the crystals of a mixture by hand, starting from an aqueous solution of the sodium ammonium salt of racemate tartaric acid. Pasteur benefited from the fact that ammonium tartrate salt that gives enantiomeric crystals with distinct crystal forms (at 77 °F). Reasoning from the macroscopic scale down to the molecular, he reckoned that the molecules had to have non-superimposable mirror images. A sample with only a single enantiomer is an ''enantiomerically pure'' or ''enantiopure'' compound. Etymology From racemic acid found in grapes; from Latin ''racemus'', meani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural polymers (such as proteins). In polymer chemistry "cross-linking" usually refers to the use of cross-links to promote a change in the polymers' physical properties. When "crosslinking" is used in the biological field, it refers to the use of a probe to link proteins together to check for protein–protein interactions, as well as other creative cross-linking methodologies. Although the term is used to refer to the "linking of polymer chains" for both sciences, the extent of crosslinking and specificities of the crosslinking agents vary greatly. As with all science, there are overlaps, and the following delineations are a starting point to understanding the subtleties. Polymer chemistry Crosslinking is the general term for the process of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |