|
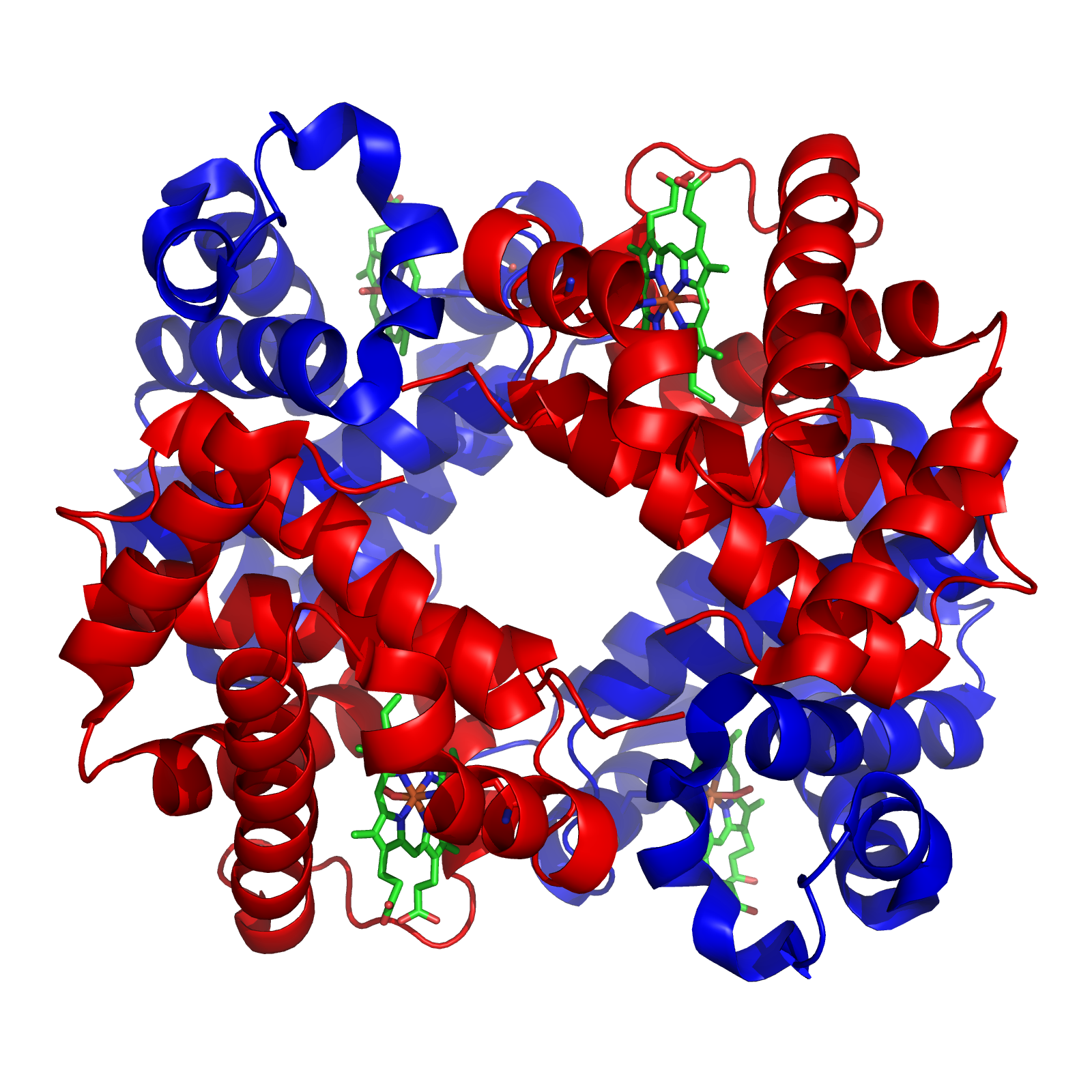
Cystathionine-β-lyase
Cystathionine beta-lyase (), also commonly referred to as CBL or β-cystathionase, is an enzyme that primarily catalyzes the following α,β-elimination reaction Thus, the substrate of this enzyme is L-cystathionine, whereas its 3 products are homocysteine, pyruvate, and ammonia. Found in plants, bacteria, and yeast, cystathionine beta-lyase is an essential part of the methionine biosynthesis pathway as homocysteine can be directly converted into methionine by methionine synthase. The enzyme belongs to the γ-family of PLP-dependent enzymes due to its use of a pyridoxal-5'-phosphate (PLP) cofactor to cleave cystathionine. The enzyme also belongs to the family of lyases, specifically the class of carbon-sulfur lyases. The systematic name of this enzyme class is L-cystathionine L-homocysteine-lyase (deaminating; pyruvate-forming). This enzyme participates in 5 metabolic pathways: methionine metabolism, cysteine metabolism, selenoamino acid metabolism, nitrogen metaboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine Metabolism
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the protonated fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methionine
Methionine (symbol Met or M) () is an essential amino acid in humans. As the precursor of other amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine plays a critical role in the metabolism and health of many species, including humans. It is encoded by the codon AUG. Methionine is also an important part of angiogenesis, the growth of new blood vessels. Supplementation may benefit those suffering from copper poisoning. Overconsumption of methionine, the methyl group donor in DNA methylation, is related to cancer growth in a number of studies. Methionine was first isolated in 1921 by John Howard Mueller. Biochemical details Methionine (abbreviated as Met or M; encoded by the codon AUG) is an α-amino acid that is used in the biosynthesis of proteins. It contains a carboxyl group (which is in the deprotonated −COO− form under biological pH conditions), an amino group (which is in the protonated fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridoxal Phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependent activities, corresponding to ~4% of all classified activities. The versatility of PLP arises from its ability to covalently bind the substrate, and then to act as an electrophilic catalyst, thereby stabilizing different types of carbanionic reaction intermediates. Role as a coenzyme PLP acts as a coenzyme in all transamination reactions, and in certain decarboxylation, deamination, and racemization reactions of amino acids. The aldehyde group of PLP forms a Schiff-base linkage (internal aldimine) with the ε-amino group of a specific lysine group of the aminotransferase enzyme. The α-amino group of the amino acid substrate displaces the ε-amino group of the active-site lysine residue in a process known as transaldimination. The resulting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex. Large assemblies of proteins such as viruses often use a small number of types of protein subunits as building blocks. A subunit is often named with a Greek or Roman letter, and the numbers of this type of subunit in a protein is indicated by a subscript. For example, ATP synthase has a type of subunit called α. Three of these are present in the ATP synthase molecule, leading to the designation α3. Larger groups of subunits can also be specified, like α3β3-hexamer and c-ring. Naturally-occurring proteins that have a relatively small number of subunits are referred to as oligomeric.Quote: ''Oligomer molecule: A molecule of intermediate relative molecular mass, the structure of which essentially comprises a small plurality of units derived, actually or conceptually, from molecules of lower relative molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol. In biochemistry, it similarly refers to a biomolecule formed of four units, that are the same (homotetramer), i.e. as in Concanavalin A or different (heterotetramer), i.e. as in hemoglobin. Hemoglobin has 4 similar sub-units while immunoglobulins have 2 very different sub-units. The different sub-units may have each their own activity, such as binding biotin in avidin tetramers, or have a common biological property, such as the allosteric binding of oxygen in hemoglobin. See also * Cluster chemistry; atomic and molecular clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Metabolism
Sulfur is metabolism, metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur is redox, reduced or redox, oxidized by organisms in a variety of forms. The chemical element, element is present in proteins, organosulfate, sulfate esters of polysaccharides, steroids, phenols, and sulfur-containing coenzymes. Oxidation Reduced sulfur compounds are oxidized by most organisms, including higher animals and higher plants. Some organisms can conserve energy (i.e., produce adenosine triphosphate, ATP) from the oxidation of sulfur. Sulfur is the sole energy source for some lithotrophic bacteria and archaea. Reduced sulfur compounds, such as hydrogen sulfide, elemental sulfur, sulfite, thiosulfate, and various polythionates (e.g., tetrathionate), are used by various lithotrophic bacteria and are all oxidized by ''Acidithiobacillus''. Sulfur oxidizers use enzymes such as Sulfide:quinone reductase, sulfur dioxygenase and sulfite oxidase to oxidize sulfur compoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Metabolism
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmospheric, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in wastewater have dramatically altered the global nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine Metabolism
Cysteine metabolism refers to the biological pathways that consume or create cysteine. The pathways of different amino acids and other metabolites interweave and overlap to creating complex systems.cysteine is metabolism creating complex systems Human cysteine metabolism In human cysteine metabolism, L-cysteine is consumed in several ways as shown below. L-Cysteine is also consumed in methionine and glutathione metabolism as well as pantothenate/coenzyme A biosynthesis. L-Cysteine is the product of several processes as well. In addition to the reactions below, L-cysteine is also a product of glycine, serine, and threonine metabolism. See also * D-cysteine desulfhydrase * Sulfur metabolism Sulfur is metabolism, metabolized by all organisms, from bacteria and archaea to plants and animals. Sulfur is redox, reduced or redox, oxidized by organisms in a variety of forms. The chemical element, element is present in proteins, organosulfate ... {{DEFAULTSORT:Cysteine Metabolism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |