|
Cross Dehydrogenative Coupling
Cross dehydrogenative coupling (also known as CDC reaction), coined by Chao-Jun Li of McGill University, is a type of coupling reaction allowing the construction of a carbon–carbon bond or C-Heteroatom bond directly from C-H bonds in the presence of an oxidant, leading to the thermodynamically unfavorable formal removal of a H2 molecule. As such, CDC are couplings belonging to the C-H activation strategy. The key to the CDC coupling is eliminating the need for substrate prefunctionalization. Therefore, the CDC reaction has the advantages of high efficiency, Atom economy and environmental friendliness. Such reactions can be achieved or activated by transition-metal catalysis or oxidation reaction (e.g. benzoquinone, peroxides, O2, hypervalent iodine), or by either photocatalysis or electrocatalysis. The mechanism and reactivity of the CDC reactions varies dramatically depending on the substrate. CDC reactions have been used to construct bonds between sp3-sp3, sp3-sp2, sp3- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chao-Jun Li
Chao-Jun "C.-J." Li is E. B. Eddy Professor of Chemistry and Canada Research Chair in Green Chemistry at McGill University, Montréal. He works on organic transformation applied to Green chemistry, including C-H activation, reactions in water and photochemistry. Education C.-J. Li was born in 1963, and obtained his BSc from Zhengzhou University (1979–1983), and completed his MSc. in organic synthesis at the Chinese Academy of Sciences (1985–1988) with Prof. T.H. Chan. He then moved to McGill University (Montréal, Québec) to do his PhD (1989–1992) with Prof. T.H. Chan again (and discovered the indium-mediated allylation reaction in water), along with Prof. David Harpp (working on organosulfur/selenium/tellurium chemistry), and went on a NSERC-funded postdoc with Prof. Barry Trost at Stanford University in the United States (1992–1994) (and discovered the so-called phosphine-catalyzed γ-addition reaction). Career and research C.-J. Li started as an assistant profess ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coupling Reactions
A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon-carbon bond in the product R-R'. The most common type of coupling reaction is the cross coupling reaction. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed cross coupling reactions. Broadly speaking, two types of coupling reactions are recognized: *Heterocouplings combine two different partners, such as in the Heck reaction of an alkene (RC=CH) and an alkyl halide (R'-X) to give a substituted alkene, or the Corey–House synthesis of an alkane by the reaction of a lithium diorganylcuprate (R2CuLi) with an organyl (pseudo)halide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Footnotes
A note is a string of text placed at the bottom of a page in a book or document or at the end of a chapter, volume, or the whole text. The note can provide an author's comments on the main text or citations of a reference work in support of the text. Footnotes are notes at the foot of the page while endnotes are collected under a separate heading at the end of a chapter, volume, or entire work. Unlike footnotes, endnotes have the advantage of not affecting the layout of the main text, but may cause inconvenience to readers who have to move back and forth between the main text and the endnotes. In some editions of the Bible, notes are placed in a narrow column in the middle of each page between two columns of biblical text. Numbering and symbols In English, a footnote or endnote is normally flagged by a superscripted number immediately following that portion of the text the note references, each such footnote being numbered sequentially. Occasionally, a number between brack ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Coupling
Oxidative coupling in chemistry is a coupling reaction of two molecular entities through an oxidative process. Usually oxidative couplings are catalysed by a transition metal complex like in classical cross-coupling reactions, although the underlying mechanism is different due to the oxidation process that requires an external (or internal) oxidant. Many such couplings utilize dioxygen as the stoichiometric oxidant but proceed by electron transfer. C-C Couplings Many oxidative couplings generate new C-C bonds. Early examples involve coupling of terminal alkynes: :2 RC≡CH + 2 Cu(I) → RC≡C-C≡CR + 2 Cu + 2 H+ Coupling of methane Coupling reactions involving methane are highly sought, related to C1 chemistry because C2 derivatives are far more valuable than methane. The oxidative coupling of methane gives ethylene: : 2 + → + 2 Aromatic coupling In oxidative aromatic coupling the reactants are electron-rich aromatic compounds. Typical substrates are phenols a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross-coupling Reaction
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = main group center) reacts with an organic halide of the type R'-X with formation of a new carbon–carbon bond in the product R-R'. Cross-coupling reaction are a subset of coupling reactions. It is often used in arylations. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism The mechanism generally involves reductive elimination of the organic substituents R and R' on a metal complex of the type LnMR(R') (where L is some arbitrary spectator ligand). The crucial intermediate LnMR(R') is formed in a two step process from a low valence precursor Ln. The oxidative addition of an organic halide (RX) to LnM gives LnMR(X). Subsequ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypervalent Iodine
Iodane generally refers to any organic derivative of iodine. Without modifier, ''iodane'' is the systematic name for the parent hydride of iodine, HI. Thus, any organoiodine compound with general formula RI (e.g., iodomethane , or iodobenzene ) is a substituted iodane. However, as used in the context of organic synthesis, the term ''iodane'' more specifically refers to organoiodine compounds with nonstandard bond order of bonds between iodine and other atoms, i.e., bond order of iodine greater than 1, making this term a synonym for hypervalent iodine. These iodine compounds are hypervalent because the iodine atom formally contains more than the 8 electrons in the valence shell required for the octet rule. When iodine is ligated to an organic residue and electronegative ligands (e.g. halides or carboxylates), hypervalent iodine occurs in a +3 oxidation state as iodine(III) or λ3-iodane, or in a +5 oxidation state as iodine(V) or λ5-iodane, or in a +7 oxidation state as iodine(V ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
McGill University
McGill University (french: link=no, Université McGill) is an English-language public research university located in Montreal, Quebec, Canada. Founded in 1821 by royal charter granted by King George IV,Frost, Stanley Brice. ''McGill University, Vol. I. For the Advancement of Learning, 1801–1895.'' McGill-Queen's University Press, 1980. the university bears the name of James McGill, a Scottish merchant whose bequest in 1813 formed the university's precursor, University of McGill College (or simply, McGill College); the name was officially changed to McGill University in 1885. McGill's main campus is on the slope of Mount Royal in downtown Montreal in the borough of Ville-Marie, with a second campus situated in Sainte-Anne-de-Bellevue, west of the main campus on Montreal Island. The university is one of two members of the Association of American Universities located outside the United States, alongside the University of Toronto, and is the only Canadian member of the Glob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
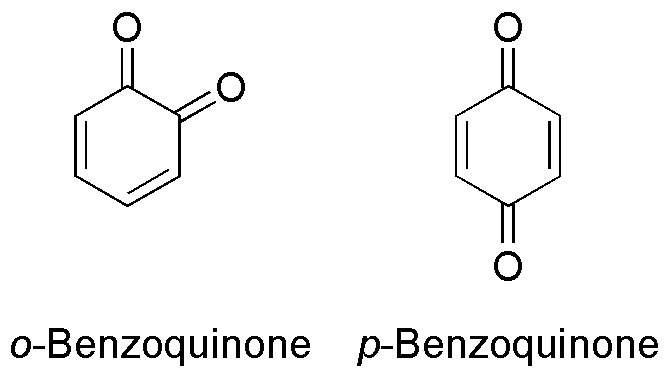
Benzoquinone
Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones: * 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone) * 1,2-Benzoquinone, less commonly, left image (also ''ortho''-benzoquinone, ''o''-benzoquinone, ''ortho''-quinone) *1,3-benzoquinone "does not exist, because its structure would be nonplanar and highly strained", though derivatives are known. An alkylated ''p''-benzoquinone has been found in the rhizomes of ''Iris kemaonensis''. See also * Arene substitution pattern Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * I ... References {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |