|
Coumestan
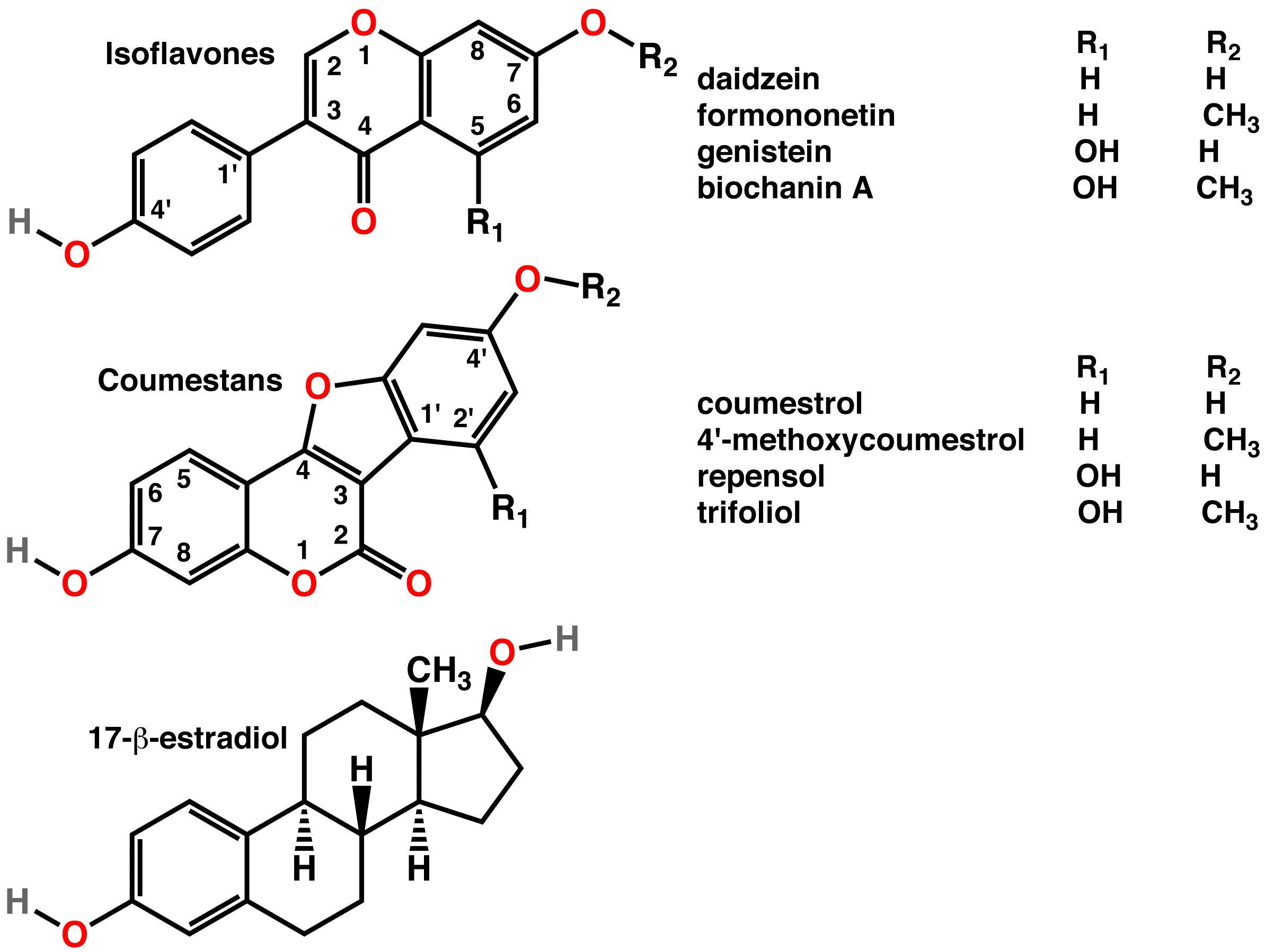
Coumestan is a heterocyclic organic compound. Coumestan forms the central core of a variety of natural compounds known collectively as coumestans. Coumestans are oxidation products of pterocarpan that are similar to coumarin. Coumestans, including coumestrol, a phytoestrogen, are found in a variety of plants. Food sources high in coumestans include split peas, pinto beans, lima beans, and especially alfalfa and clover sprouts. Coumestrol has about the same binding affinity for the ER-β estrogen receptor as 17β-estradiol, but much less affinity than 17α-estradiol, although the estrogenic potency of coumestrol at both receptors is much less than that of 17β-estradiol. Because of the estrogenic activity of some coumestans, a variety of syntheses have been developed that allow the preparation of coumestans so that their pharmacological effects can be explored. Coumestans Image:coumestrol.png, Coumestrol Coumestrol is a natural organic compound in the class of phytoche ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plicadin
Plicadin is a coumestan Coumestan is a heterocyclic organic compound. Coumestan forms the central core of a variety of natural compounds known collectively as coumestans. Coumestans are oxidation products of pterocarpan that are similar to coumarin. Coumestans, includ ... found in the herb '' Psoralea plicata''.Nazli Rasool, Abdul Qasim Khan, Viqar Uddin Ahmad and Abdul Malik (1991)A benzoquinone and a coumestan from Psoralea plicata. ''Phytochemistry'', Volume 30, Issue 8, pp. 2800-2803. References Coumestans Phenols {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytoestrogen
A phytoestrogen is a plant-derived xenoestrogen (see estrogen) not generated within the endocrine system, but consumed by eating plants or manufactured foods. Also called a "dietary estrogen", it is a diverse group of naturally occurring nonsteroidal plant compounds that, because of its structural similarity with estradiol (17-β-estradiol), have the ability to cause estrogenic or antiestrogenic effects. Phytoestrogens are not essential nutrients because their absence from the diet does not cause a disease, nor are they known to participate in any normal biological function. Common foods containing phytoestrogens are soy protein, beans, oats, barley, rice, coffee, apples, carrots (see Food Sources section below for bigger list). Its name comes from the Greek ''phyto'' ("plant") and ''estrogen'', the hormone which gives fertility to female mammals. The word "estrus" - Greek οίστρος - means "sexual desire", and "gene" - Greek γόνο - is "to generate". It has been hypothe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coumestrol
Coumestrol is a natural organic compound in the class of phytochemicals known as coumestans. Coumestrol was first identified as a compound with estrogenic properties by E. M. Bickoff in ladino clover and alfalfa in 1957. It has garnered research interest because of its estrogenic activity and prevalence in some foods, including soybeans, brussels sprouts, spinach and a variety of legumes. The highest concentrations of coumestrol are found in clover, Kala Chana (a type of chick pea), and Alfalfa sprouts. Coumestrol is a phytoestrogen, mimicking the biological activity of estrogens. Phytoestrogens are able to pass through cell membranes due to their low molecular weight and stable structure, and they are able to interact with the enzymes and receptors of cells. Coumestrol binds to the ERα and ERβ with similar affinity to that of estradiol (94% and 185% of the relative binding affinity of estradiol at the ERα and ERβ, respectively), although the estrogenic activity of coume ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wedelolactone
Wedelolactone is an organic chemical compound classified as a coumestan that occurs in '' Eclipta alba'' (false daisy) and in '' Wedelia calendulacea''. References Catechols Coumestans Phenol ethers {{aromatic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coumestrol
Coumestrol is a natural organic compound in the class of phytochemicals known as coumestans. Coumestrol was first identified as a compound with estrogenic properties by E. M. Bickoff in ladino clover and alfalfa in 1957. It has garnered research interest because of its estrogenic activity and prevalence in some foods, including soybeans, brussels sprouts, spinach and a variety of legumes. The highest concentrations of coumestrol are found in clover, Kala Chana (a type of chick pea), and Alfalfa sprouts. Coumestrol is a phytoestrogen, mimicking the biological activity of estrogens. Phytoestrogens are able to pass through cell membranes due to their low molecular weight and stable structure, and they are able to interact with the enzymes and receptors of cells. Coumestrol binds to the ERα and ERβ with similar affinity to that of estradiol (94% and 185% of the relative binding affinity of estradiol at the ERα and ERβ, respectively), although the estrogenic activity of coume ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alfalfa
Alfalfa () (''Medicago sativa''), also called lucerne, is a perennial flowering plant in the legume family Fabaceae. It is cultivated as an important forage crop in many countries around the world. It is used for grazing, hay, and silage, as well as a green manure and cover crop. The name alfalfa is used in North America. The name lucerne is the more commonly used name in the United Kingdom, South Africa, Australia, and New Zealand. The plant superficially resembles clover (a cousin in the same family), especially while young, when trifoliate leaves comprising round leaflets predominate. Later in maturity, leaflets are elongated. It has clusters of small purple flowers followed by fruits spiralled in 2 to 3 turns containing 10–20 seeds. Alfalfa is native to warmer temperate climates. It has been cultivated as livestock fodder since at least the era of the ancient Greeks and Romans. Etymology The word ''alfalfa'' is a Spanish modification of the Arabic word ''al-faṣfa� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alfatradiol
Alfatradiol, also known as 17α-estradiol and sold under the brand names Avicis, Avixis, Ell-Cranell Alpha, and Pantostin, is a weak estrogen and 5α-reductase inhibitor medication which is used topically in the treatment of pattern hair loss (androgenic alopecia or pattern baldness) in men and women. It is a stereoisomer of the endogenous steroid hormone and estrogen 17β-estradiol (or simply estradiol). Medical uses Alfatradiol is used in form of an ethanolic solution for topical application on the scalp. Similarly to other drugs against alopecia, topical or oral, it has to be applied continuously to prevent further hair loss. Regrowth of hair that was already lost is only possible to a limited extent. In general, advanced alopecia does not respond well to medical treatment, which has historically been thought to be a consequence of the hair roots being lost. A university-led study (including several authors who are advisors to companies such as Pfizer) in 103 women compari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estradiol
Estradiol (E2), also spelled oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is responsible for the development of female secondary sexual characteristics such as the breasts, widening of the hips and a female-associated pattern of fat distribution. It is also important in the development and maintenance of female reproductive tissues such as the mammary glands, uterus and vagina during puberty, adulthood and pregnancy. It also has important effects in many other tissues including bone, fat, skin, liver, and the brain. Though estradiol levels in males are much lower than in females, estradiol has important roles in males as well. Apart from humans and other mammals, estradiol is also found in most vertebrates and crustaceans, insects, fish, and other animal species. Estradiol is produced especially within the follicles of the ovaries, but also in o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Receptor
Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen ( 17β-estradiol). Two classes of ER exist: nuclear estrogen receptors (ERα and ERβ), which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs) (GPER (GPR30), ER-X, and Gq-mER), which are mostly G protein-coupled receptors. This article refers to the former (ER). Once activated by estrogen, the ER is able to translocate into the nucleus and bind to DNA to regulate the activity of different genes (i.e. it is a DNA-binding transcription factor). However, it also has additional functions independent of DNA binding. As hormone receptors for sex steroids (steroid hormone receptors), ERs, androgen receptors (ARs), and progesterone receptors (PRs) are important in sexual maturation and gestation. Proteomics There are two different forms of the estrogen receptor, usually referred to as α a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen Receptor Beta
Estrogen receptor beta (ERβ) also known as NR3A2 (nuclear receptor subfamily 3, group A, member 2) is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by the ''ESR2'' gene. Function ERβ is a member of the family of estrogen receptors and the superfamily of nuclear receptor transcription factors. The gene product contains an N-terminal DNA binding domain and C-terminal ligand binding domain and is localized to the nucleus, cytoplasm, and mitochondria. Upon binding to 17-β-estradiol, estriol or related ligands, the encoded protein forms homo-dimers or hetero-dimers with estrogen receptor α that interact with specific DNA sequences to activate transcription. Some isoforms dominantly inhibit the activity of other estrogen receptor family members. Several alternatively spliced transcript variants of this gene have been described, but the full-length nature of some of these variants h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and furan are quinol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |