|
Clinical Data Acquisition
Acquisition or collection of clinical trial data can be achieved through various methods that may include, but are not limited to, any of the following: paper or electronic medical records, paper forms completed at a site, interactive voice response systems, local electronic data capture systems, or central web based systems. There is arguably no more important document than the instrument that is used to acquire the data from the clinical trial with the exception of the protocol, which specifies the conduct of that clinical trial. The quality of the data collected relies first and foremost on the quality of that instrument. No matter how much time and effort go into conducting the clinical trial, if the correct data points were not collected, a meaningful analysis may not be possible. It follows, therefore, that the design, development and quality assurance of such an instrument must be given the utmost attention. The ICH guidelines on good clinical practice (GCP) use the term � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Data Capture
An electronic data capture (EDC) system is a computerized system designed for the collection of clinical data in electronic format for use mainly in human clinical trials. EDC replaces the traditional paper-based data collection methodology to streamline data collection and expedite the time to market for drugs and medical devices. EDC solutions are widely adopted by pharmaceutical companies and contract research organizations (CRO). Typically, EDC systems provide: * a graphical user interface component for data entry * a validation component to check user data * a reporting tool for analysis of the collected data EDC systems are used by life sciences organizations, broadly defined as the pharmaceutical, medical device and biotechnology industries in all aspects of clinical research, but are particularly beneficial for late-phase (phase III-IV) studies and pharmacovigilance and post-market safety surveillance. EDC can increase data accuracy and decrease the time to collect data ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Title 21 CFR Part 11
Title 21 CFR Part 11 is the part of Title 21 of the Code of Federal Regulations that establishes the United States Food and Drug Administration (FDA) regulations on electronic records and electronic signatures (ERES). Part 11, as it is commonly called, defines the criteria under which electronic records and electronic signatures are considered trustworthy, reliable, and equivalent to paper records (Title 21 CFR Part 11 Section 11.1 (a)). Coverage Practically speaking, Part 11 applies to drug makers, medical device manufacturers, biotech companies, biologics developers, CROs, and other FDA-regulated industries, with some specific exceptions. It requires that they implement controls, including audits, system validations, audit trails, electronic signatures, and documentation for software and systems involved in processing the electronic data that FDA predicate rules require them to maintain. A predicate rule is any requirement set forth in the Federal Food, Drug and Cosmetic Act, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematized Nomenclature Of Medicine
The Systematized Nomenclature of Medicine (SNOMED) is a systematic, computer-processable collection of medical terms, in human and veterinary medicine, to provide codes, terms, synonyms and definitions which cover anatomy, diseases, findings, procedures, microorganisms, substances, etc. It allows a consistent way to index, store, retrieve, and aggregate medical data across specialties and sites of care. Although now international, SNOMED was started in the U.S. by the College of American Pathologists (CAP) in 1973 and revised into the 1990s. In 2002 CAP's SNOMED Reference Terminology (SNOMED RT) was merged with, and expanded by, the National Health Service's Clinical Terms Version 3 (previously known as the Read codes) to produce SNOMED CT. Versions of SNOMED released prior to 2001 were based on a multiaxial, hierarchical classification system. As in any such system, a disease may be located in a body organ (anatomy), which results in a code in a topography axis and may lead ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Decisions
Health, according to the World Health Organization, is "a state of complete physical, mental and social well-being and not merely the absence of disease and infirmity".World Health Organization. (2006)''Constitution of the World Health Organization''– ''Basic Documents'', Forty-fifth edition, Supplement, October 2006. A variety of definitions have been used for different purposes over time. Health can be promoted by encouraging healthful activities, such as regular physical exercise and adequate sleep, and by reducing or avoiding unhealthful activities or situations, such as smoking or excessive stress. Some factors affecting health are due to individual choices, such as whether to engage in a high-risk behavior, while others are due to structural causes, such as whether the society is arranged in a way that makes it easier or harder for people to get necessary healthcare services. Still, other factors are beyond both individual and group choices, such as genetic disorders. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patient-reported Outcome
A patient-reported outcome (PRO) is a health outcome directly reported by the patient who experienced it. It stands in contrast to an outcome reported by someone else, such as a physician-reported outcome, a nurse-reported outcome, and so on. PRO methods, such as questionnaires, are used in clinical trials or other clinical settings, to help better understand a treatment's efficacy or effectiveness. The use of digitized PROs, or electronic patient-reported outcomes (ePROs), is on the rise in today's health research setting. Terminology PROs should not be confused with PCOs, or ''patient-centered outcomes''. The latter implies the use of a questionnaire covering issues and concerns that are specific to a patient. Instead, ''patient-reported'' outcomes refers to reporting situations in which only the patient provides information related to a specific treatment or condition; this information may or may not be of concern to the patient. Further, PROs should not be confused with PR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Level 7
Health Level Seven or HL7 refers to a set of international standards for transfer of clinical and administrative data between software applications used by various healthcare providers. These standards focus on the application layer, which is "layer 7" in the OSI model. The HL7 standards are produced by Health Level Seven International, an international standards organization, and are adopted by other standards issuing bodies such as American National Standards Institute and International Organization for Standardization. Hospitals and other healthcare provider organizations typically have many different computer systems used for everything from billing records to patient tracking. All of these systems should communicate with each other (or "interface") when they receive new information, or when they wish to retrieve information, but not all do so. HL7 International specifies a number of flexible standards, guidelines, and methodologies by which various healthcare systems ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
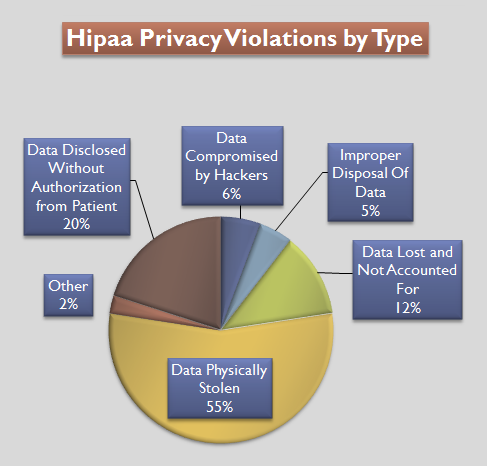
Health Insurance Portability And Accountability Act
The Health Insurance Portability and Accountability Act of 1996 (HIPAA or the Kennedy– Kassebaum Act) is a United States Act of Congress enacted by the 104th United States Congress and signed into law by President Bill Clinton on August 21, 1996. It modernized the flow of healthcare information, stipulates how personally identifiable information maintained by the healthcare and healthcare insurance industries should be protected from fraud and theft, and addressed some limitations on healthcare insurance coverage. It generally prohibits healthcare providers and healthcare businesses, called ''covered entities'', from disclosing protected information to anyone other than a patient and the patient's authorized representatives without their consent. With limited exceptions, it does not restrict patients from receiving information about themselves. It does not prohibit patients from voluntarily sharing their health information however they choose, nor does it require confidentiali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Directive 95/46/EC On The Protection Of Personal Data
The Data Protection Directive, officially Directive 95/46/EC, enacted in October 1995, is a European Union directive which regulates the processing of personal data within the European Union (EU) and the free movement of such data. The Data Protection Directive is an important component of EU privacy and human rights law. The principles set out in the Data Protection Directive are aimed at the protection of fundamental rights and freedoms in the processing of personal data. The General Data Protection Regulation, adopted in April 2016, superseded the Data Protection Directive and became enforceable on 25 May 2018. Context The right to privacy is a highly developed area of law in Europe. All the member states of the Council of Europe (CoE) are also signatories of the European Convention on Human Rights (ECHR). Article 8 of the ECHR provides a right to respect for one's "private and family life, his home and his correspondence", subject to certain restrictions. The European ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Data Capture
An electronic data capture (EDC) system is a computerized system designed for the collection of clinical data in electronic format for use mainly in human clinical trials. EDC replaces the traditional paper-based data collection methodology to streamline data collection and expedite the time to market for drugs and medical devices. EDC solutions are widely adopted by pharmaceutical companies and contract research organizations (CRO). Typically, EDC systems provide: * a graphical user interface component for data entry * a validation component to check user data * a reporting tool for analysis of the collected data EDC systems are used by life sciences organizations, broadly defined as the pharmaceutical, medical device and biotechnology industries in all aspects of clinical research, but are particularly beneficial for late-phase (phase III-IV) studies and pharmacovigilance and post-market safety surveillance. EDC can increase data accuracy and decrease the time to collect data ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Data Management
Data management comprises all disciplines related to handling data as a valuable resource. Concept The concept of data management arose in the 1980s as technology moved from sequential processing (first punched cards, then magnetic tape) to random access storage. Since it was now possible to store a discrete fact and quickly access it using random access disk technology, those suggesting that data management was more important than business process management used arguments such as "a customer's home address is stored in 75 (or some other large number) places in our computer systems." However, during this period, random access processing was not competitively fast, so those suggesting "process management" was more important than "data management" used batch processing time as their primary argument. As application software evolved into real-time, interactive usage, it became obvious that both management processes were important. If the data was not well defined, the data ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Document Architecture
The HL7 Clinical Document Architecture (CDA) is an XML-based markup standard intended to specify the encoding, structure and semantics of clinical documents for exchange. In November 2000, HL7 published Release 1.0. The organization published Release 2.0 with its "2005 Normative Edition." Content CDA specifies the syntax and supplies a framework for specifying the full semantics of a clinical document, defined by six characteristics: # Persistence # Stewardship # Potential for authentication # Context # Wholeness # Human readability CDA can hold any kind of clinical information that would be included in a patient's medical record; examples include: * Discharge summary (following inpatient care) * History & physical * Specialist reports, such as those for medical imaging or pathology An XML element in a CDA supports unstructured text, as well as links to composite documents encoded in pdf, docx, or rtf, as well as image formats like jpg and png. It was developed using the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)