|
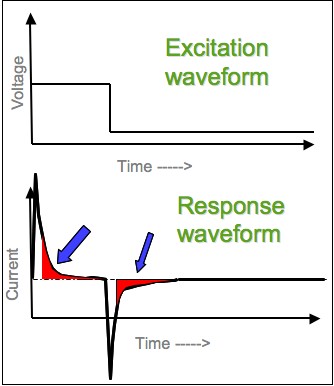
Chronoamperometry
Chronoamperometry is an electrochemical technique in which the potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode (caused by the potential step) is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells this decay is much slower than the charging decay-cells with n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cottrell Equation
In electrochemistry, the Cottrell equation describes the change in electric current with respect to time in a controlled potential experiment, such as chronoamperometry. Specifically it describes the current response when the potential is a step function in time. It was derived by Frederick Gardner Cottrell in 1903. For a simple redox event, such as the ferrocene/ferrocenium couple, the current measured depends on the rate at which the analyte diffuses to the electrode. That is, the current is said to be "diffusion controlled." The Cottrell equation describes the case for an electrode that is planar but can also be derived for spherical, cylindrical, and rectangular geometries by using the corresponding Laplace operator and boundary conditions in conjunction with Fick's second law of diffusion.Bard, A. J.; Faulkner, L. R. “Electrochemical Methods. Fundamentals and Applications” 2nd Ed. Wiley, New York. 2001. : i = \frac where, : i = current, in units of A : n = numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Skin Conductance
Electrochemical skin conductance (ESC) is an objective, non-invasive and quantitative electrophysiological measure. It is based on reverse iontophoresis and (multiple) steady chronoamperometry (more specifically chronovoltametry). ESC is intended to provide insight into and assess sudomotor (or sweat gland) function and small fiber peripheral neuropathy. Description of the ESC measurement Currently, ESC measurement can be obtained with the use of a medical device, called Sudoscan. No specific patient preparation or medical personnel training is required. The measure lasts less than 3 minutes, and is innocuous and non-invasive. The apparatus consists of stainless-steel electrodes for the hands and the feet which are connected to a computer for recording and data management purposes. To conduct an ESC test, the patients place their hands and feet on the electrodes. Sweat glands are most numerous on the palms of the hands and soles of the feet, and thus well suited for sud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Working Electrode
The working electrode is the electrode in an electrochemical system on which the reaction of interest is occurring. The working electrode is often used in conjunction with an auxiliary electrode, and a reference electrode in a three electrode system. Depending on whether the reaction on the electrode is a reduction or an oxidation, the working electrode is called cathodic or anodic, respectively. Common working electrodes can consist of materials ranging from inert metals such as gold, silver or platinum, to inert carbon such as glassy carbon, boron doped diamond or pyrolytic carbon, and mercury drop and film electrodes. Chemically modified electrodes are employed for the analysis of both organic and inorganic samples. Special types * Ultramicroelectrode (UME) * Rotating disk electrode (RDE) * Rotating ring-disk electrode (RRDE) * Hanging mercury drop electrode (HMDE) * Dropping mercury electrode (DME) See also * Auxiliary electrode * Electrochemical cell * Electrochemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroanalytical Methods
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The four main categories are potentiometry (the difference in electrode potentials is measured), amperometry (electric current is the analytical signal), coulometry (charge passed during a certain time is recorded), and voltammetry (the cell's current is measured while actively altering the cell's potential). Potentiometry Potentiometry passively measures the potential of a solution between two electrodes, affecting the solution very little in the process. One electrode is called the reference electrode and has a constant potential, while the other one is an indicator electrode whose potential changes with the sample's composition. Theref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalic Acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus ''Oxalis'', commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous. Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula . History The preparation of salts of oxalic acid (crab acid) from plants had been known, at least since 1745, when the Dutch botanist and physician Herman Boerhaave isolated a salt from wood sorrel. By 1773, François Pierre Savary of Fribourg, Switzerland had isolated oxalic acid from i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polarography
Polarography is a type of voltammetry where the working electrode is a dropping mercury electrode (DME) or a static mercury drop electrode (SMDE), which are useful for their wide cathodic ranges and renewable surfaces. It was invented in 1922 by Czechs, Czech chemist Jaroslav Heyrovský, for which he won the Nobel prize in 1959. The main advantages of mercury as electrode material are as follows: 1) a large voltage window: ca. from +0.2 V to -1.8 V vs reversible hydrogen electrode (RHE). Hg electrode is particularly well-suited for studying electroreduction reactions. 2) very reproducible electrode surface, since mercury is liquid. 3) very easy cleaning of the electrode surface by making a new drop of mercury from a large Hg pool connected by a glass capillary. Polarography played a major role as an experimental tool in the advancement of both Analytical Chemistry and Electrochemistry until the 1970s, when it was supplanted by other methods, that did not require the use of mercury ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E And T
E, or e, is the fifth letter and the second vowel letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''e'' (pronounced ); plural ''ees'', ''Es'' or ''E's''. It is the most commonly used letter in many languages, including Czech, Danish, Dutch, English, French, German, Hungarian, Latin, Latvian, Norwegian, Spanish, and Swedish. History The Latin letter 'E' differs little from its source, the Greek letter epsilon, 'Ε'. This in turn comes from the Semitic letter '' hê'', which has been suggested to have started as a praying or calling human figure ('' hillul'' 'jubilation'), and was most likely based on a similar Egyptian hieroglyph that indicated a different pronunciation. In Semitic, the letter represented (and in foreign words); in Greek, ''hê'' became the letter epsilon, used to represent . The various forms of the Old Italic script and the Latin alph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Voltammetry Cell
Cycle, cycles, or cyclic may refer to: Anthropology and social sciences * Cyclic history, a theory of history * Cyclical theory, a theory of American political history associated with Arthur Schlesinger, Sr. * Social cycle, various cycles in social sciences ** Business cycle, the downward and upward movement of gross domestic product (GDP) around its ostensible, long-term growth trend Arts, entertainment, and media Films * ''Cycle'' (2008 film), a Malayalam film * ''Cycle'' (2017 film), a Marathi film Literature * ''Cycle'' (magazine), an American motorcycling enthusiast magazine * Literary cycle, a group of stories focused on common figures Music Musical terminology * Cycle (music), a set of musical pieces that belong together **Cyclic form, a technique of construction involving multiple sections or movements **Interval cycle, a collection of pitch classes generated from a sequence of the same interval class **Song cycle, individually complete songs designed to be performe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |