|
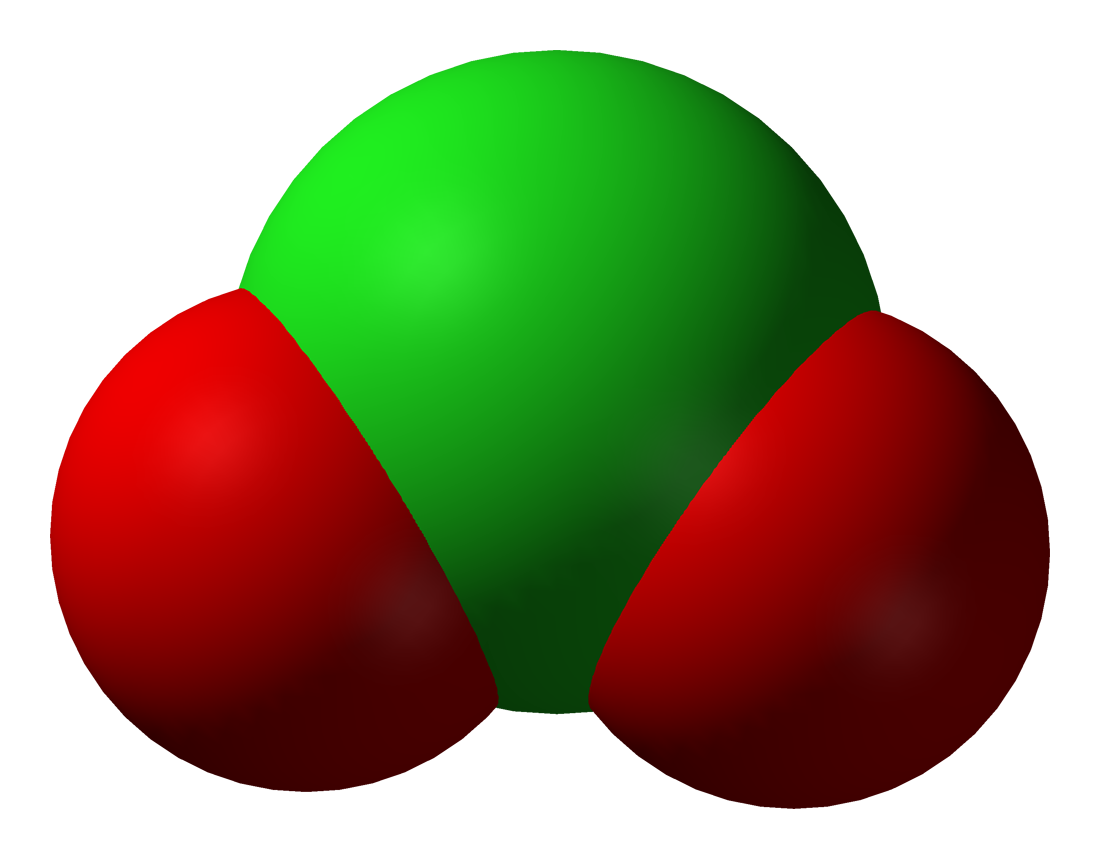
Chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead. Structure and bonding The chlorate ion cannot be satisf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a Roman numeral in parentheses, e.g. chlorate (VII), refers to a particular oxyanion of chlorine. As predicted by valence shell electron pair repulsion theory, chlorate anions have trigonal pyramidal structures. Chlorates are powerful oxidizers and should be kept away from organics or easily oxidized materials. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, charcoal, organic solvents, metals, etc.) will readily deflagrate. Chlorates were once widely used in pyrotechnics for this reason, though their use has fallen due to their instability. Most pyrotechnic applications that formerly used chlorates now use the more stable perchlorates instead. Structure and bonding The chlorate ion cannot be satisf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Chlorate
Sodium chlorate is an inorganic compound with the chemical formula Na ClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper. Synthesis Industrially, sodium chlorate is produced by the electrolysis of concentrated sodium chloride solutions. All other processes are obsolete. The sodium chlorate process is not to be confused with the chloralkali process, which is an industrial process for the electrolytic production of sodium hydroxide and chlorine gas. The overall reaction can be simplified to the equation: First, chloride is oxidised to form intermediate hypochlorite, ClO−, which undergoes further oxidisation to chlorate along two competing reaction paths: (1) Anodic chlorate formation at the boundary layer between the electrolyte and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Chlorate
Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used * in fireworks, propellants and explosives, * to prepare oxygen, both in the lab and in chemical oxygen generators, * as a disinfectant, for example in medical mouthwashes, * in agriculture as an herbicide. Production On the industrial scale, potassium chlorate is produced by the salt metathesis reaction of sodium chlorate and potassium chloride: : NaClO3 + KCl → NaCl + KClO3 The reaction is driven by the low solubility of potassium chlorate in water. The equilibrium of the reaction is shifted to the right hand side by the continuo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloric Acid
Chloric acid, H Cl O3, is an oxoacid of chlorine, and the formal precursor of chlorate salts. It is a strong acid ( p''K''a ≈ −2.7 (''***note: pKa not in agreement with properties in chem box at right'')) and oxidizing agent. Properties Chloric acid is thermodynamically unstable with respect to disproportionation. Chloric acid is stable in cold aqueous solution up to a concentration of approximately 30%, and solution of up to 40% can be prepared by careful evaporation under reduced pressure. Above these concentrations, chloric acid solutions decompose to give a variety of products, for example: :8 HClO3 → 4 HClO4 + 2 H2O + 2 Cl2 + 3 O2 :3 HClO3 → HClO4 + H2O + 2 ClO2 Hazards Chloric acid is a powerful oxidizing agent. Most organics and flammables will deflagrate on contact. Production It can be prepared by the reaction of sulfuric acid with barium chlorate, the insoluble barium sulfate being removed by precipitation: :Ba(ClO3)2 + H2SO4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, . The majority of perchlorates are commercially produced salts. They are mainly used as oxidizers for pyrotechnic devices and to control static electricity in food packaging. Perchlorate contamination in food, water, and other parts of the environment has been studied in the U.S. because of harmful effects on human health. Perchlorate ions are somewhat toxic to the thyroid gland. Most perchlorates are colorless solids that are soluble in water. Four perchlorates are of primary commercial interest: ammonium perchlorate , perchloric acid , potassium perchlorate and sodium perchlorate . Perchlorate is the anion resulting from the dissociation of perchloric acid and its salts upon their dissolution in water. Many perchlorate salts are soluble in non-aqueous solutions. Production Perchlorate salts are produced industrially by the oxidation of aqueous solutions of sodium chlorate by electrolysis. This method is used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Chlorate
Magnesium chlorate is an inorganic chemical consisting of a magnesium cation and two chlorate anions: its chemical formula is Mg(ClO3)2. Production Magnesium chlorate was first produced in 1920 by reacting magnesium oxide with chlorine gas to produce a mixture of magnesium chloride and magnesium chlorate. They were not able to separate the magnesium chlorate from the magnesium chloride. Other production methods were reported such as reacting chlorine gas with magnesium hydroxide or magnesium carbonate to create magnesium hypochlorite then converting the hypochlorite to the chlorate. But, not a lot of studies have been done on the properties of magnesium chlorate. The most modern method is converting magnesium chloride electrochemically: :MgCl2 + 6 H2O + e− → Mg(ClO3)2 + 6 H2 After, the magnesium chlorate was separated from the magnesium chloride by using the solubility of magnesium chlorate in acetone Acetone (2-propanone or dimethyl ketone), is an organic compound with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypochlorite
In chemistry, hypochlorite is an anion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of bleaching powder, swimming pool "chlorine"). The Cl-O distance in ClO− is 1.69 Å. The name can also refer to esters of hypochlorous acid, namely organic compounds with a ClO– moiety (chemistry), group covalent bond, covalently bound to the rest of the molecule. The principal example is tert-butyl hypochlorite, which is a useful chlorinating agent. Most hypochlorite salts are handled as aqueous solutions. Their primary applications are as bleaching, disinfection, and water treatment agents. They are also used in chemistry for Halogenation, chlorination and oxidation reactions. Reactions Acid reaction Acidification of hypochlorites generates hypochlorous acid, which exists in an equilibrium with chlorine. A high pH drives the reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorite
The chlorite ion, or chlorine dioxide anion, is the halite with the chemical formula of . A chlorite (compound) is a compound that contains this group, with chlorine in the oxidation state of +3. Chlorites are also known as salts of chlorous acid. Compounds The free acid, chlorous acid HClO2, is the least stable oxoacid of chlorine and has only been observed as an aqueous solution at low concentrations. Since it cannot be concentrated, it is not a commercial product. The alkali metal and alkaline earth metal compounds are all colorless or pale yellow, with sodium chlorite (NaClO2) being the only commercially important chlorite. Heavy metal chlorites (Ag+, Hg+, Tl+, Pb2+, and also Cu2+ and ) are unstable and decompose explosively with heat or shock. Sodium chlorite is derived indirectly from sodium chlorate, NaClO3. First, the explosively unstable gas chlorine dioxide, ClO2 is produced by reducing sodium chlorate with a suitable reducing agent such as methanol, hydrogen perox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups. Hydroxide ion The hydroxide ion is a natural par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |