|
Calcium Lactate
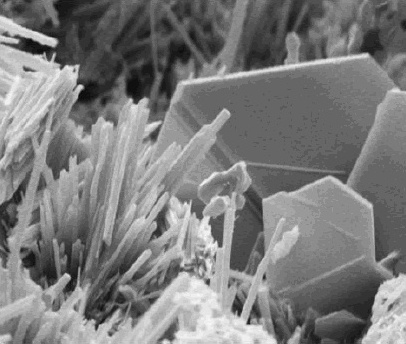
Calcium lactate is a white crystalline salt with formula , consisting of two lactate anions (CHOH) for each calcium cation . It forms several hydrates, the most common being the pentahydrate ·5. Calcium lactate is used in medicine, mainly to treat calcium deficiencies; and as a food additive with E number of E327. Some cheese crystals consist of calcium lactate. Properties The lactate ion is chiral, with two enantiomers, D (−,''R'') and L (+,''S''). The L isomer is the one normally synthesized and metabolized by living organisms, but some bacteria can produce the D form or convert the L to D. Thus calcium lactate also has D and L isomers, where all anions are of the same type. Some synthesis processes yield a mixture of the two in equal parts, resulting in the DL (racemic) salt. Both the L and the DL forms occur as crystals on the surface of aging Cheddar cheese.G.F. Tansman, P.S. Kindstedt, J.M. Hughes (2014): "Powder X-ray diffraction can differentiate between enantiom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efflorescent
In chemistry, efflorescence (which means "to flower out" in French) is the migration of a salt to the surface of a porous material, where it forms a coating. The essential process involves the dissolving of an internally held salt in water, or occasionally in another solvent. The water, with the salt now held in solution, migrates to the surface, then evaporates, leaving a coating of the salt. In what has been described as "primary efflorescence", the water is the invader and the salt was already present internally, and a reverse process, where the salt is originally present externally and is then carried inside in solution, is referred to as "secondary efflorescence". Efflorescences can occur in natural and built environments. On porous construction materials it may present a cosmetic outer problem only (primary efflorescence causing staining), but can sometimes indicate internal structural weakness (migration/degradation of component materials). Efflorescence may clog the po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram
The gram (originally gramme; SI unit symbol g) is a Physical unit, unit of mass in the International System of Units (SI) equal to one one thousandth of a kilogram. Originally defined as of 1795 as "the absolute weight of a volume of pure water equal to Cube (algebra), the cube of the hundredth part of a metre [1 Cubic centimetre, cm3], and at Melting point of water, the temperature of Melting point, melting ice", the defining temperature (~0 °C) was later changed to 4 °C, the temperature of maximum density of water. However, by the late 19th century, there was an effort to make the Base unit (measurement), base unit the kilogram and the gram a derived unit. In 1960, the new International System of Units defined a ''gram'' as one one-thousandth of a kilogram (i.e., one gram is Scientific notation, 1×10−3 kg). The kilogram, 2019 redefinition of the SI base units, as of 2019, is defined by the International Bureau of Weights and Measures from the fixed numeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parathyroidectomy
Parathyroidectomy is the surgical removal of one or more of the (usually) four parathyroid glands. This procedure is used to remove an adenoma or hyperplasia of these glands when they are producing excessive parathyroid hormone (PTH): hyperparathyroidism. The glands are usually four in number and located adjacent to the posterior surface of the thyroid gland, but their exact location is variable. When an elevated PTH level is found, a sestamibi scan or an ultrasound may be performed in order to confirm the presence and location of abnormal parathyroid tissue. Indications The main indication for parathyroidectomy is primary hyperparathyroidism, a condition in which one or more of the parathyroid glands produce excessive parathyroid hormone. Not all cases of primary hyperparathyroidism require surgery, but it is recommended if the condition causes significant symptoms or if it affects the kidneys (nephrocalcinosis) or bone health (osteoporosis), and also in people under 50 even if t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parathyroid
Parathyroid glands are small endocrine glands in the neck of humans and other tetrapods. Humans usually have four parathyroid glands, located on the back of the thyroid gland in variable locations. The parathyroid gland produces and secretes parathyroid hormone in response to a low blood calcium, which plays a key role in regulating the amount of calcium in the blood and within the bones. Parathyroid glands share a similar blood supply, venous drainage, and lymphatic drainage to the thyroid glands. Parathyroid glands are derived from the epithelial lining of the third and fourth pharyngeal pouches, with the superior glands arising from the fourth pouch and the inferior glands arising from the higher third pouch. The relative position of the inferior and superior glands, which are named according to their final location, changes because of the migration of embryological tissues. Hyperparathyroidism and hypoparathyroidism, characterized by alterations in the blood calcium levels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetany
Tetany or tetanic seizure is a medical sign consisting of the involuntary contraction of muscles, which may be caused by disorders that increase the action potential frequency of muscle cells or the nerves that innervate them. Muscle cramps caused by the disease tetanus are not classified as tetany; rather, they are due to a lack of inhibition to the neurons that supply muscles. Tetanic contractions (physiologic tetanus) are a broad range of muscle contraction types, of which tetany is only one. Signs and symptoms Tetany is characterized by contraction of distal muscles of the hands (carpal spasm with extension of interphalangeal joints and adduction and flexion of the metacarpophalangeal joints) and feet (pedal spasm) and is associated with tingling around the mouth and distally in the limbs. Causes * The usual cause of tetany is a deficiency of calcium. An excess of phosphate (high phosphate-to-calcium ratio) can also trigger the spasms. * Underfunction of the parathyroid gla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Citrate
Calcium citrate is the calcium salt of citric acid. It is commonly used as a food additive ( E333), usually as a preservative, but sometimes for flavor. In this sense, it is similar to sodium citrate. Calcium citrate is also found in some dietary calcium supplements (e.g. Citracal or Caltrate). Calcium makes up 24.1% of calcium citrate (anhydrous) and 21.1% of calcium citrate (tetrahydrate) by mass. The tetrahydrate occurs in nature as the mineral Earlandite. In 2020, calcium was the 204th most commonly prescribed medication in the United States, with more than 2million prescriptions. Chemical properties Calcium citrate is sparingly soluble in water. Needle-shaped crystals of tricalcium dicitrate tetrahydrate a3(C6H5O7)2(H2O)2�2H2O were obtained by hydrothermal synthesis. The crystal structure comprises a three-dimensional network in which eightfold coordinated Ca2+ cations are linked by citrate anions and hydrogen bonds between two non-coordinating crystal water molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antacid
An antacid is a substance which neutralizes stomach acidity and is used to relieve heartburn, indigestion or an upset stomach. Some antacids have been used in the treatment of constipation and diarrhea. Marketed antacids contain salts of aluminum, calcium, magnesium, or sodium. Some preparations contain a combination of two salts, such as magnesium carbonate and aluminium hydroxide (e.g. hydrotalcite). Medical uses Antacids are available over the counter and are taken by mouth to quickly relieve occasional heartburn, the major symptom of gastroesophageal reflux disease and indigestion. Treatment with antacids alone is symptomatic and only justified for minor symptoms.U.S. Department of Health & Human Services. Agency for Healthcare Research and Quality 23 September 201Consumer Summary – Treatment Options for GERD or Acid Reflux Disease: A Review of the Research for Adults Alternative uses for antacids include constipation, diarrhea, hyperphosphatemia, and urinary alkalizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Veterinary Medicine
Veterinary medicine is the branch of medicine that deals with the prevention, management, diagnosis, and treatment of disease, disorder, and injury in animals. Along with this, it deals with animal rearing, husbandry, breeding, research on nutrition, and product development. The scope of veterinary medicine is wide, covering all animal species, both domesticated and wild, with a wide range of conditions that can affect different species. Veterinary medicine is widely practiced, both with and without professional supervision. Professional care is most often led by a veterinary physician (also known as a veterinarian, veterinary surgeon, or "vet"), but also by paraveterinary workers, such as veterinary nurses or technicians. This can be augmented by other paraprofessionals with specific specialties, such as animal physiotherapy or dentistry, and species-relevant roles such as farriers. Veterinary science helps human health through the monitoring and control of zoonotic disease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate
In organic chemistry, a carbohydrate () is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula (where ''m'' may or may not be different from ''n''), which does not mean the H has covalent bonds with O (for example with , H has a covalent bond with C but not with O). However, not all carbohydrates conform to this precise stoichiometric definition (e.g., uronic acids, deoxy-sugars such as fucose), nor are all chemicals that do conform to this definition automatically classified as carbohydrates (e.g. formaldehyde and acetic acid). The term is most common in biochemistry, where it is a synonym of saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Monosaccharides and disaccharides, the smallest (lower molecular wei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermentation
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology. In microorganisms, fermentation is the primary means of producing adenosine triphosphate (ATP) by the degradation of organic nutrients anaerobically. Humans have used fermentation to produce foodstuffs and beverages since the Neolithic age. For example, fermentation is used for preservation in a process that produces lactic acid found in such sour foods as pickled cucumbers, kombucha, kimchi, and yogurt, as well as for producing alcoholic beverages such as wine and beer. Fermentation also occurs within the gastrointestinal tracts of all a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide (portlandite) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)