|
Cycloprop-2-ene Carboxylic Acid
Cycloprop-2-ene carboxylic acid is a mycotoxin found in some mushrooms such as '' Russula subnigricans''. When ingested, the molecule is known to cause rhabdomyolysis. In mice, the oral of this molecule is 2.5 mg/kg and poisoning is indicated by an increase in serum creatine phosphokinase activity. Polymerization via the ene reaction In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ... abolishes toxicity. 3-(Cycloprop-2-en-1-oyl)oxazolidinones are a class of ‘unusually stable’ derivatives of cycloprop-2-ene carboxylic acid that have been synthesized by Fox ''et al''. As mentioned by Fox ''et'' ''al'', this class of ‘unusually stable’ derivatives are dienophiles when involved in a Diels-Alder reaction. References {{Reflist Carboxylic acids Cyclopropenes Mycotoxin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycotoxin
A mycotoxin (from the Greek μύκης , "fungus" and τοξίνη , "toxin") is a toxic secondary metabolite produced by organisms of kingdom Fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops. Examples of mycotoxins causing human and animal illness include aflatoxin, citrinin, fumonisins, ochratoxin A, patulin, trichothecenes, zearalenone, and ergot alkaloids such as ergotamine. One mold species may produce many different mycotoxins, and several species may produce the same mycotoxin. Production Most fungi are aerobic (use oxygen) and are found almost everywhere in extremely small quantities due to the diminute size of their spores. They consume organic matter wherever humidity and temperature are sufficient. Where conditions are right, fungi proliferate into colonies and mycotoxin levels become high. The reason for the product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Russula Subnigricans
''Russula subnigricans'', known as nisekurohatsu (Japanese), is a basidiomycete mushroom of the genus ''Russula'' found in China, Japan, and Taiwan. Description The flesh turns pale red when cut, but doesn't turn black unlike '' Russula nigricans''. The species was named by Japanese mycologist Tsuguo Hongo in 1955. The name was formerly applied to the North American fungus ''Russula eccentrica'' in California. It has been reclassified as ''Russula cantharellicola'', where it grows in association with coast live oak (''Quercus agrifolia'') trees in California oak woodland habitats. Toxicity ''Russula subnigricans'' is a poisonous mushroom, and has been responsible for mushroom poisoning in Taiwan and Japan. The effect is a serious one, rhabdomyolysis. The toxins responsible are the very unusual cycloprop-2-ene carboxylic acid (a toxic molecule consisting of only four carbon atoms) and Russuphelin A (a heavily chlorinated polyphenolic). See also * List of deadly fungi Althou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhabdomyolysis
Rhabdomyolysis (also called rhabdo) is a condition in which damaged skeletal muscle breaks down rapidly. Symptoms may include muscle pains, weakness, vomiting, and confusion. There may be tea-colored urine or an irregular heartbeat. Some of the muscle breakdown products, such as the protein myoglobin, are harmful to the kidneys and can cause acute kidney injury. The muscle damage is mostly caused by a crush injury, strenuous exercise, medications, or a substance use disorder. Other causes include infections, electrical injury, heat stroke, prolonged immobilization, lack of blood flow to a limb, or snake bites. Statins (prescription drugs to lower cholesterol) are considered a small risk. Some people have inherited muscle conditions that increase the risk of rhabdomyolysis. The diagnosis is supported by a urine test strip which is positive for "blood" but the urine contains no red blood cells when examined with a microscope. Blood tests show a creatine kinase activity greate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Creatine Phosphokinase
Creatine kinase (CK), also known as creatine phosphokinase (CPK) or phosphocreatine kinase, is an enzyme () expressed by various tissues and cell types. CK catalyses the conversion of creatine and uses adenosine triphosphate (ATP) to create phosphocreatine (PCr) and adenosine diphosphate (ADP). This CK enzyme reaction is reversible and thus ATP can be generated from PCr and ADP. In tissues and cells that consume ATP rapidly, especially skeletal muscle, but also brain, photoreceptor cells of the retina, hair cells of the inner ear, spermatozoa and smooth muscle, PCr serves as an energy reservoir for the rapid buffering and regeneration of ATP ''in situ'', as well as for intracellular energy transport by the PCr shuttle or circuit. Thus creatine kinase is an important enzyme in such tissues. Clinically, creatine kinase is assayed in blood tests as a marker of damage of CK-rich tissue such as in myocardial infarction (heart attack), rhabdomyolysis (severe muscle breakdown), muscular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple free-radical reaction, radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. Alkanes can also be polymerized, but only with the help of strong acids. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
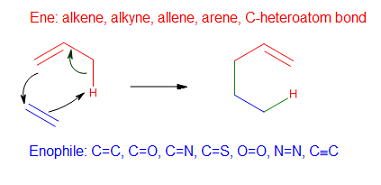
Ene Reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position. This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Many useful Lewis acid-catalyzed ene reactions have been also developed, which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products. Ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed +2cycloaddition with Woodward–Hoffmann symbol π4s_+_π2s.html" ;"title="sub>π4s + π2s">sub>π4s + π2s It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |