|
Cyclopentadienyltungsten Tricarbonyl Dimer
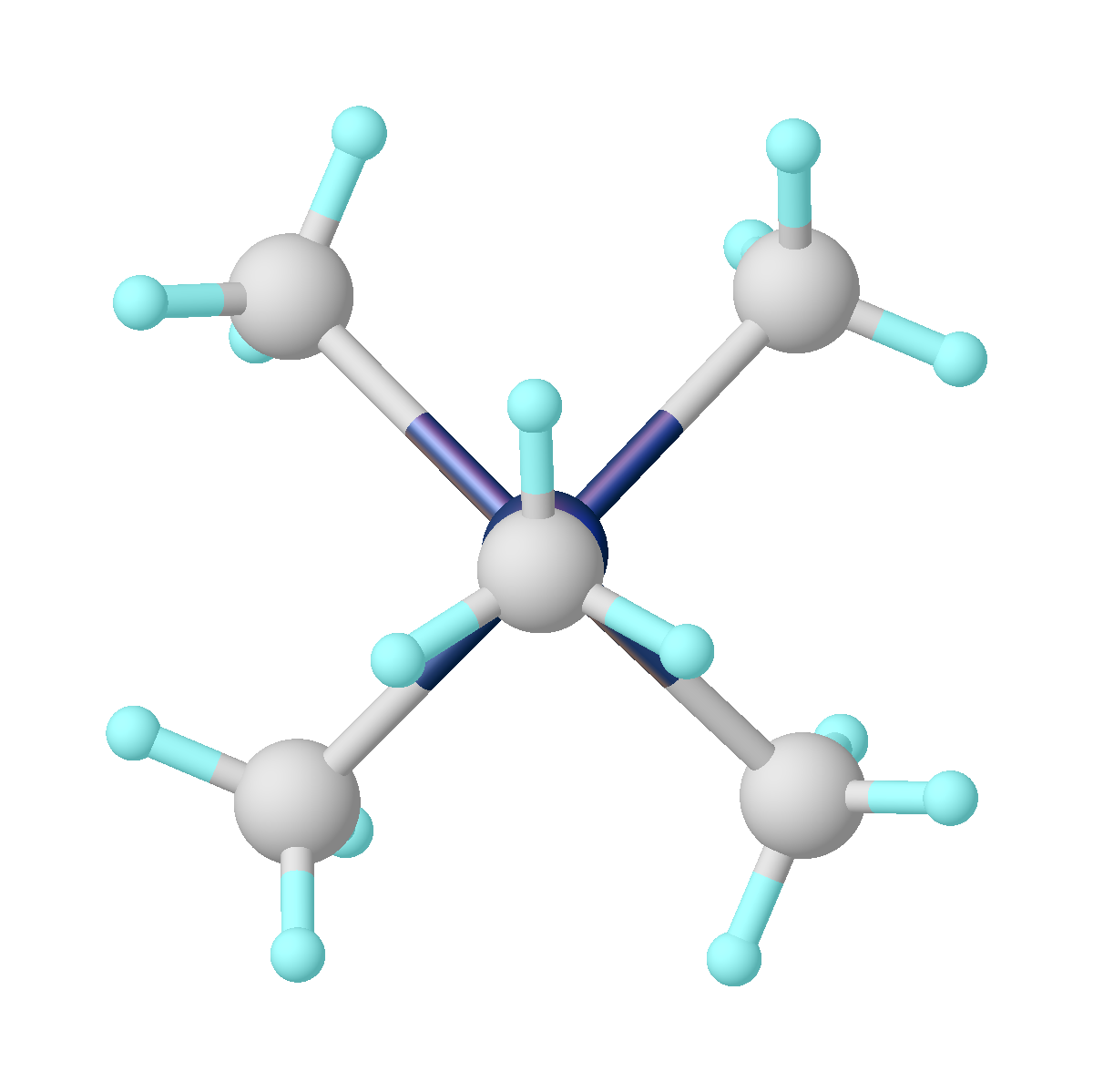
Cyclopentadienyltungsten tricarbonyl dimer is the organotungsten compound with the formula Cp2W2(CO)6, where Cp is C5H5. A dark red crystalline solid, it is the subject of research, although it has no or few practical uses. Structure and synthesis The molecule exists in two rotamers, gauche and anti. The six CO ligands are terminal, and the W-W bond distance is 3.222 Å. The compound is prepared by treatment of tungsten hexacarbonyl with sodium cyclopentadienide followed by oxidation of the resulting NaW(CO)3(C5H5). Related compounds * Cyclopentadienylmolybdenum tricarbonyl dimer * Cyclopentadienylchromium tricarbonyl dimer Cyclopentadienylchromium tricarbonyl dimer is the organochromium compound with the formula Cp2Cr2(CO)6, where Cp is C5H5. A dark green crystalline solid. It is the subject of research it exists in measureable equilibrium quantities with the mono ... References {{tungsten compounds Organotungsten compounds Carbonyl complexes Dimers (chemistry) Half ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotamer
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted just by rotations about formally single bonds (refer to figure on single bond rotation). While any two arrangements of atoms in a molecule that differ by rotation about single bonds can be referred to as different conformations, conformations that correspond to local minima on the potential energy surface are specifically called conformational isomers or conformers. Conformations that correspond to local maxima on the energy surface are the transition states between the local-minimum conformational isomers. Rotations about single bonds involve overcoming a rotational energy barrier to interconvert one conformer to another. If the energy barrier is low, there is free rotation and a sample of the compound exists as a rapidly equilibrating mixture of multiple conformers; if the energy barrier is high enough then there is restricted rotation, a molecule may exist for a relati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Sheffield
, mottoeng = To discover the causes of things , established = – University of SheffieldPredecessor institutions: – Sheffield Medical School – Firth College – Sheffield Technical School – University College of Sheffield , type = Public research university , academic_staff = 5,670 (2020) - including academic atypical staff , administrative_staff = , chancellor = Lady Justice Rafferty , vice_chancellor = Koen Lamberts , students = () , undergrad = () , postgrad = () , endowment = £46.7 million (2021) , budget = £741.0 million (2020–21) , city = Sheffield , state = South Yorkshire , country = England , coor = , campus = Urban , colours = Black & gold , affiliations = Russell Group WUN ACUN8 Group White Rose Sutton 30EQUISAMBAUniversities UK , website = , logo = The University of Sheffield (informally Sheffield University or TUOS) is a public research university in Sheffield, South Yorkshire, England. Its history traces back to the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten Hexacarbonyl
Tungsten hexacarbonyl (also called tungsten carbonyl) is the chemical compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.Kubas, G. J., Metal Dihydrogen and σ-Bond Complexes, Kluwer Academic/Plenum Publishers: New York, 2001. This colorless compound, like its chromium and molybdenum analogs, is noteworthy as a volatile, air-stable derivative of tungsten in its zero oxidation state. Preparation, properties, and structure W(CO)6 is prepared by the reduction of tungsten hexachloride under a pressure of carbon monoxide. The compound is relatively air-stable. It is sparingly soluble in nonpolar organic solvents. Tungsten carbonyl is widely used in electron beam-induced deposition technique - it is easily vaporized and decomposed by the electron beam providing a convenient source of tungsten atoms. W(CO)6 adopts an octahedral geometry consisting of six rod-like CO ligands radiating from the central W atom with dipole moment 0 D. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyclopentadienide
Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities. Preparation Sodium cyclopentadienide is commercially available as a solution in THF. It is prepared by treating cyclopentadiene with sodium: : The conversion can be conducted by heating a suspension of molten sodium in dicyclopentadiene.Tarun K. Panda, Michael T. Gamer, Peter W. Roesky "An Improved Synthesis of Sodium and Potassium Cyclopentadienide" Organometallics, 2003, 22, 877–878. In former times, the sodium was provided in the form of "sodium wire" or "sodium sand", a fine dispersion of sodium prepared by melting sodium in refluxing xylene and rapidly stirring. Sodium hydride is a convenient base: : In early work, Grignard reagents were used as bases. With a p''K''a of 15, cyclopen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Syntheses
''Inorganic Syntheses'' is a book series which aims to publish "detailed and foolproof" procedures for the synthesis of inorganic compounds. Although this series of books are edited, they usually are referenced like a journal, without mentioning the names of the checkers (referees) or the editor. A similar format is usually followed for the series ''''. Volumes See also *Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. I ...
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienylmolybdenum Tricarbonyl Dimer
Cyclopentadienylmolybdenum tricarbonyl dimer is the chemical compound with the formula Cp2Mo2(CO)6, where Cp is C5H5. A dark red solid, it has been the subject of much research although it has no practical uses. Structure and synthesis The molecule exists in two rotamers, gauche and anti. The six CO ligands are terminal and the Mo-Mo bond distance is 3.2325 Å. The compound is prepared by treatment of molybdenum hexacarbonyl with sodium cyclopentadienide followed by oxidation of the resulting NaMo(CO)3(C5H5). Other methods have been developed starting with Mo(CO)3(CH3CN)3 instead of Mo(CO)6. Reactions Thermolysis of this compound in hot solution of diglyme (bis(2-methoxyethyl)ether) results in decarbonylation, giving the tetracarbonyl, which has a formal triple bond between the Mo centers (''d''MoMo = 2.448 Å):Cotton, F. A.; Walton, R. A. "Multiple Bonds Between Metal Atoms" Oxford (Oxford): 1993, p 564ff. . :(C5H5)2Mo2(CO)6 → (C5H5)2Mo2(CO)4 + 2 CO The resulting cyclopenta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienylchromium Tricarbonyl Dimer
Cyclopentadienylchromium tricarbonyl dimer is the organochromium compound with the formula Cp2Cr2(CO)6, where Cp is C5H5. A dark green crystalline solid. It is the subject of research it exists in measureable equilibrium quantities with the monometallic radical CpCr(CO)3. Structure and synthesis The six CO ligands are terminal, and the Cr-Cr bond distance is 3.281 Å, 0.06 Å longer than the related dimolybdenum compound. The compound is prepared by treatment of chromium hexacarbonyl with sodium cyclopentadienide Sodium cyclopentadienide is an organosodium compound with the formula C5H5Na. The compound is often abbreviated as NaCp, where Cp− is the cyclopentadienide anion. Sodium cyclopentadienide is a colorless solid, although samples often are p ... followed by oxidation of the resulting NaCr(CO)3(C5H5). Related compounds * Cyclopentadienylmolybdenum tricarbonyl dimer * Cyclopentadienyltungsten tricarbonyl dimer References {{chromium compounds Organochromium c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotungsten Compounds
Organomolybdenum chemistry is the chemistry of chemical compounds with Mo-C bonds. The heavier group 6 elements molybdenum and tungsten form organometallic compounds similar to those in organochromium chemistry but higher oxidation states tend to be more common. Mo(0) and more reduced states Molybdenum hexacarbonyl is the precursor to many substituted derivatives. It reacts with organolithium reagents to give anionic acyls which can be O-alkylated to give Fischer carbenes. file:(Mesitylene)molybdenum tricarbonyl.png, 144px, Structure of (Mesitylene)molybdenum tricarbonyl, (mesitylene)molybdenum tricarbonyl. Mo(CO)6 reacts with arenes to give piano-stool complexes such as (Mesitylene)molybdenum tricarbonyl, (mesitylene)molybdenum tricarbonyl. Cycloheptatrienemolybdenum tricarbonyl, which is related to (arene)Mo(CO)3, reacts with trityl salts to give the cycloheptatrienyl complex: :(C7H8)Mo(CO)3 + (C6H5)3C+ → [(C7H7)Mo(CO)3]+ + (C6H5)3CH file:CHTMo(CO)3.png, 144px, Structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl Complexes
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. A sp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimers (chemistry)
Dimer may refer to: * Dimer (chemistry), a chemical structure formed from two similar sub-units ** Protein dimer, a protein quaternary structure ** d-dimer * Dimer model, an item in statistical mechanics, based on ''domino tiling'' * Julius Dimer Julius Dimer (1 August 1871 – 20 October 1945) was a German chess master. At the beginning of his career, he played in several mini tournaments (''Quadrangular'') in Germany; at Altona 1897, Elmshorn 1898, Munich 1900, Kiel 1901, Hamburg 1903, ... (1871–1945), German chess master See also * Dimery (botany), having two parts in a distinct whorl of a plant structure * Di (other), a prefix * Dymer (other) * -mer, a suffix * Oligomer * Peierls transition, sometimes called dimerization {{Disambiguation, surname ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |