|
Conductive Agent
Conductive agents are used to ensure electrode, electrodes have good charge and discharge performance. Usually, a certain amount of conductive material is added during the production of the pole piece, and the micro current is collected between the active material and the current collector to reduce the micro current. The contact resistance of the electrode accelerates the rate of movement of electrons, and at the same time, can effectively increase the migration rate of lithium ions in the electrode material, thereby improving the charge and discharge efficiency of the electrode. The conductive agent carbon black is used for improving the conductivity of the electrodes and decreasing the resistance of interaction. Carbon black conductive agent The conductive carbon black is characterized by small particle size, particularly large specific surface area, and particularly good electrical conductivity, and it can function as a liquid absorption and liquid retention in the battery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. The electrophore, invented by Johan Wilcke, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery made was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes separated by brine-soaked paper disks. Due to fluctuation in the voltage provided by the voltaic cell it wasn't very practical. The first practical battery was invented in 1839 and named the Daniell cell after John Frederic Daniell. Still making use of the zinc–copper electrode combination. Since then many more batteries have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pole Piece
A pole piece is a structure composed of material of high magnetic permeability that serves to direct the magnetic field produced by a magnet. A pole piece attaches to and in a sense extends a pole of the magnet, hence the name. Pole pieces are used with both permanent magnets and electromagnets. In the case of an electromagnet, the pole piece or pieces simply extend the magnetic core and can even be regarded as part of it, particularly if they are made of the same material. In the case of a permanent magnet, the distinction between the magnet itself and the pole piece or pieces is more clear cut. Materials The traditional material for pole pieces was soft iron. While still often used with permanent magnets, soft iron suffers from eddy currents which make it less suitable for use with electromagnets, and particularly inefficient when the magnet is excited by alternating current. Shapes and forms Pole pieces take many shapes and forms depending on the application. A traditiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ScienceDirect
ScienceDirect is a website which provides access to a large bibliographic database of scientific and medical publications of the Dutch publisher Elsevier. It hosts over 18 million pieces of content from more than 4,000 academic journals and 30,000 e-books of this publisher. The access to the full-text requires subscription, while the bibliographic metadata is free to read. ScienceDirect is operated by Elsevier. It was launched in March 1997. Usage The journals are grouped into four main sections: ''Physical Sciences and Engineering'', ''Life Sciences'', ''Health Sciences'', and ''Social Sciences and Humanities''. Article abstracts are freely available, and access to their full texts (in PDF and, for newer publications, also HTML) generally requires a subscription or pay-per-view purchase unless the content is freely available in open access. Subscriptions to the overall offering hosted on ScienceDirect, rather than to specific titles it carries, are usually acquired through a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2019 ''Journal Citation Reports'' (with an ascribed impact factor of 42.778), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in autumn 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander Macmillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the journal; ''Nature'' redoubled its efforts in exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Contact Resistance
The term contact resistance refers to the contribution to the total resistance of a system which can be attributed to the contacting interfaces of electrical leads and connections as opposed to the intrinsic resistance. This effect is described by the term electrical contact resistance (ECR) and arises as the result of the limited areas of true contact at an interface and the presence of resistive surface films or oxide layers. ECR may vary with time, most often decreasing, in a process known as resistance creep. The idea of potential drop on the injection electrode was introduced by William Shockley to explain the difference between the experimental results and the model of gradual channel approximation. In addition to the term ECR, ''interface resistance'', ''transitional resistance'', or just simply ''correction term'' are also used. The term ''parasitic resistance'' is used as a more general term, of which it is usually assumed that contact resistance is a major component. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyacetylene
Polyacetylene (IUPAC name: polyethyne) usually refers to an organic polymer with the repeating unit . The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high conductivity upon doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics (organic semiconductors). This discovery was recognized by the Nobel Prize in Chemistry in 2000. Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals". Despite the promise of this polymer in the field of conductive polymers, many of its properties such as instability to air and difficulty with processing have led t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
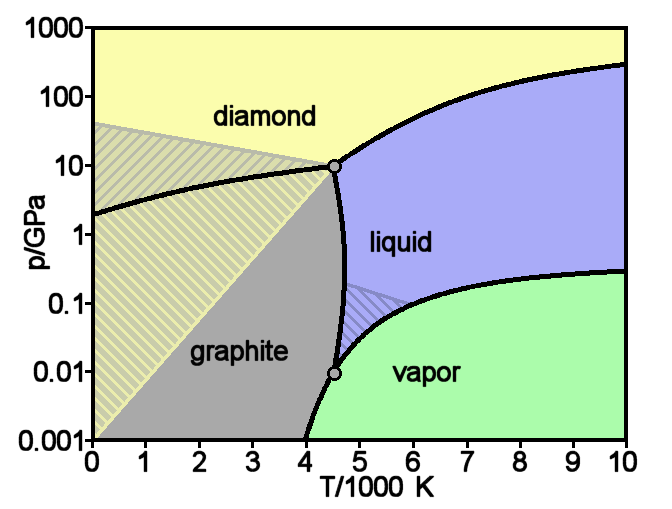
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |