|
Chromium(VI) Peroxide
Chromium(VI) peroxide or chromium oxide peroxide is an unstable compound with the formula CrO5. This compound contains one oxo ligand and two peroxo ligands, making a total of five oxygen atoms per chromium atom. Preparation and properties Chromium(VI) peroxide is formed by the addition of acidified hydrogen peroxide solutions to solutions of metal chromates or dichromates, such as sodium chromate or potassium dichromate. The generally yellow chromates or orange dichromates turn to dark blue as chromium(VI) peroxide is formed. Chromate or dichromate reacts with hydrogen peroxide and an acid to give chromium peroxide and water. :CrO42− + 2 H2O2 + 2 H+ → CrO5 + 3 H2O With this method, the chromium(VI) peroxide will decompose after a few seconds, turning green as chromium(III) compounds are formed. :2 CrO5 + 7 H2O2 + 6 H+ → 2 Cr3+ + 10 H2O + 7 O2 To avoid this decomposition, it is possible to stabilize chromium(VI) oxide peroxide in water-immiscible organic solvents su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxides
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide Zinc peroxide (ZnO2) appears as a bright yellow powder at room temperature. It was historically used as a surgical antiseptic. More recently zinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics in chemistry and related fields. They are published on YouTube and produced by Brady Haran, a former BBC video journalist, mainly featuring Sir Martyn Poliakoff, Peter Licence, Stephen Liddle, Debbie Kays, Neil Barnes, Sam Tang, and other scientists at the University of Nottingham. Development The project began recording on 9 June 2008 and the initial videos were completed on 17 July 2008. The collection includes videos, each just a few minutes long, for all 118 known elements with a video for each element, as well as many additional supplemental chemistry videos. The 118 element videos and introduction videos were all shot unscripted in June and July 2008. Since the initial videos were completed in 2008 the team has been refining and up ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Nottingham
The University of Nottingham is a public university, public research university in Nottingham, United Kingdom. It was founded as University College Nottingham in 1881, and was granted a royal charter in 1948. The University of Nottingham belongs to the research intensive Russell Group association. Nottingham's main campus (University Park Campus, Nottingham, University Park) with Jubilee Campus and teaching hospital (Queen's Medical Centre) are located within the City of Nottingham, with a number of smaller campuses and sites elsewhere in Nottinghamshire and Derbyshire. Outside the UK, the university has campuses in Semenyih, Malaysia, and Ningbo, China. Nottingham is organised into five constituent faculties, within which there are more than 50 schools, departments, institutes and research centres. Nottingham has about 45,500 students and 7,000 staff, and had an income of £694 million in 2020–21, of which £114.9 million was from research grants and contracts. The institution's ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martyn Poliakoff
Sir Martyn Poliakoff (born 16 December 1947) is a British chemist, working on gaining insights into fundamental chemistry, and on developing environmentally acceptable processes and materials. The core themes of his work are supercritical fluids, infrared spectroscopy and lasers. He is a research professor in chemistry at the University of Nottingham. His group comprises several members of staff, postdoctoral research fellows, postgraduate students and overseas visitors. As well as carrying out research at the University of Nottingham, he is a lecturer, teaching a number of modules including green chemistry. Poliakoff became popularly known in the late 2000s and early 2010s as the main presenter for the YouTube channel ''Periodic Videos''. Early life Poliakoff was born to a British-Jewish mother, Ina (''née'' Montagu), and Russian-Jewish father, Alexander Poliakoff (russian: Поляко́в). He has a younger brother, the screenwriter and director Stephen Poliakoff. His pat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraperoxochromate
Potassium peroxochromate, potassium tetraperoxochromate(V), or simply potassium perchromate, is an inorganic chemical having the chemical formula K3 r(O2)4 It is a red-brown paramagnetic solid. It is the potassium salt of tetraperoxochromate(V), one of the few examples of chromium in the +5 oxidation state and one of the rare examples of a complex stabilized only by peroxide ligands. This compound is used as a source of singlet oxygen. Preparation Potassium peroxochromate is prepared by treating potassium chromate with hydrogen peroxide Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3% ... at 0 ºC: : 2 + 8 → 2 + 8 The intermediate tetraperoxochromate(VI) is reduced by hydrogen peroxide, forming tetraperoxochromate(V): : 2 + 2 + → 2 + 2 + Thus, the overall reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2019 '' Journal Citation Reports'' (with an ascribed impact factor of 42.778), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in autumn 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander Macmillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the journal; ''Nature'' redoubled its efforts in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron (journal)
''Tetrahedron'' is a weekly peer-reviewed scientific journal covering the field of organic chemistry. According to the ''Journal Citation Reports'', ''Tetrahedron'' has a 2020 impact factor of 2.457. ''Tetrahedron'' and Elsevier Elsevier () is a Dutch academic publishing company specializing in scientific, technical, and medical content. Its products include journals such as '' The Lancet'', '' Cell'', the ScienceDirect collection of electronic journals, '' Trends'', ..., its publisher, support an annual symposium. In 2010, complaints were raised over its high subscription cost. Notable papers , the Web of Science lists ten papers from ''Tetrahedron'' that have more than 1000 citations. The four articles that have been cited more than 2000 times are: * – cited 2228 times * – cited 2162 times * – cited 2124 times * – cited 2107 times See also * '' Tetrahedron Letters'' * '' Tetrahedron Computer Methodology'' * '' Polyhedron'' (journal) Ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
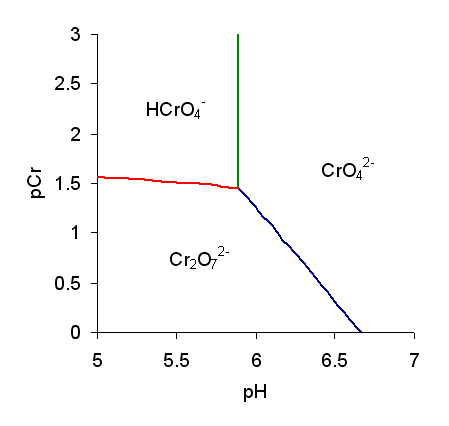
Monochromate
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, potassium chromate Potassium-dichromate-sample.jpg, potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, CrO(O2)2, is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex CrO(O2)2py. Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. :2 + 2 H+ + H2O The predominance diagram shows that the position of the equilibrium depends ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2-Orbital hybridisation, hybridized. The aldehyde group is somewhat polar molecule, polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.png)