|
Chloride Cell
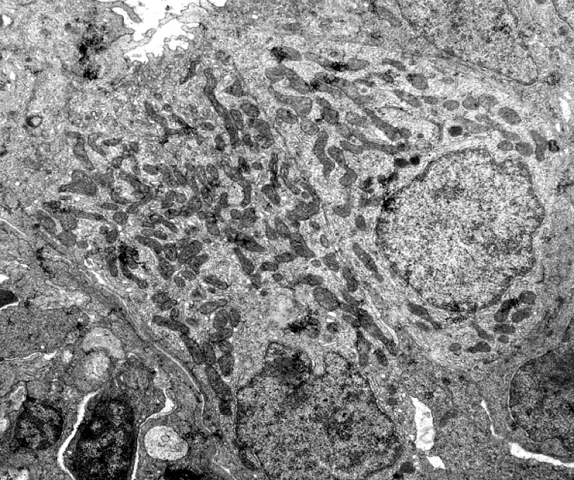
An ionocyte (formerly called a chloride cell) is a mitochondrion-rich cell within ionoregulatory organs of animals, such as teleost fish gill, insect Malpighian tubules, crustacean gills, antennal glands and maxillary glands, and copepod Crusalis organs. These cells contribute to the maintenance of optimal osmotic, ionic, and acid-base levels within metazoans. In aquatic invertebrates, ionocytes perform the functions of both ion uptake and ion excretion. In marine teleost fish, by expending energy to power the enzyme Na+/K+-ATPase and in coordination with other protein transporters, ionocytes pump excessive sodium and chloride ions against the concentration gradient into the ocean. Conversely, freshwater teleost ionocytes use this low intracellular environment to attain sodium and chloride ions into the organism, and also against the concentration gradient. In larval fishes with underdeveloped / developing gills, ionocytes can be found on the skin and fins. Mechanism of action Ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride Cell
An ionocyte (formerly called a chloride cell) is a mitochondrion-rich cell within ionoregulatory organs of animals, such as teleost fish gill, insect Malpighian tubules, crustacean gills, antennal glands and maxillary glands, and copepod Crusalis organs. These cells contribute to the maintenance of optimal osmotic, ionic, and acid-base levels within metazoans. In aquatic invertebrates, ionocytes perform the functions of both ion uptake and ion excretion. In marine teleost fish, by expending energy to power the enzyme Na+/K+-ATPase and in coordination with other protein transporters, ionocytes pump excessive sodium and chloride ions against the concentration gradient into the ocean. Conversely, freshwater teleost ionocytes use this low intracellular environment to attain sodium and chloride ions into the organism, and also against the concentration gradient. In larval fishes with underdeveloped / developing gills, ionocytes can be found on the skin and fins. Mechanism of action Ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrion
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitochondria, and one multicellular organism, '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell (biology)
The cell is the basic structural and functional unit of life forms. Every cell consists of a cytoplasm enclosed within a membrane, and contains many biomolecules such as proteins, DNA and RNA, as well as many small molecules of nutrients and metabolites.Cell Movements and the Shaping of the Vertebrate Body in Chapter 21 of Molecular Biology of the Cell '' fourth edition, edited by Bruce Alberts (2002) published by Garland Science. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos. It is also common to describe small molecules such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
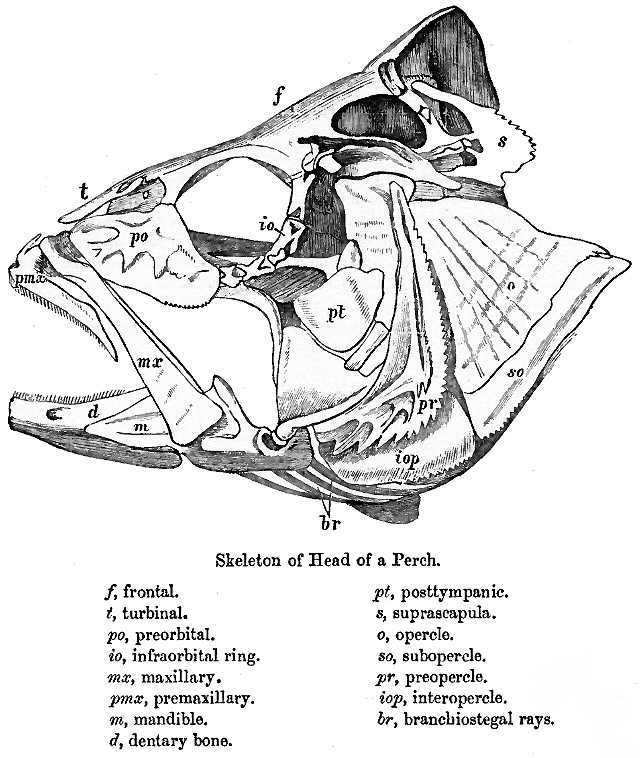
Teleost
Teleostei (; Greek ''teleios'' "complete" + ''osteon'' "bone"), members of which are known as teleosts ), is, by far, the largest infraclass in the class Actinopterygii, the ray-finned fishes, containing 96% of all extant species of fish. Teleosts are arranged into about 40 orders and 448 family (biology), families. Over 26,000 species have been described. Teleosts range from giant oarfish measuring or more, and ocean sunfish weighing over , to the minute male anglerfish ''Photocorynus spiniceps'', just long. Including not only torpedo-shaped fish built for speed, teleosts can be flattened vertically or horizontally, be elongated cylinders or take specialised shapes as in anglerfish and seahorses. The difference between teleosts and other bony fish lies mainly in their jaw bones; teleosts have a movable premaxilla and corresponding modifications in the jaw musculature which make it possible for them to cranial kinesis, protrude their jaws outwards from the mouth. This is of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fish Gill
Fish gills are organs that allow fish to breathe underwater. Most fish exchange gases like oxygen and carbon dioxide using gills that are protected under gill covers (operculum) on both sides of the pharynx (throat). Gills are tissues that are like short threads, protein structures called filaments. These filaments have many functions including the transfer of ions and water, as well as the exchange of oxygen, carbon dioxide, acids and ammonia. Each filament contains a capillary network that provides a large surface area for exchanging oxygen and carbon dioxide. Fish exchange gases by pulling oxygen-rich water through their mouths and pumping it over their gills. Within the gill filaments, capillary blood flows in the opposite direction to the water, causing counter-current exchange. The gills push the oxygen-poor water out through openings in the sides of the pharynx. Some fish, like sharks and lampreys, possess multiple gill openings. However, bony fish have a single gill op ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Na+/K+-ATPase
NA, N.A., Na, nA or n/a may refer to: Chemistry and physics * Sodium, symbol Na, a chemical element * Avogadro constant (''N''A) * Nucleophilic addition, a type of reaction in organic chemistry * Numerical aperture, a number that characterizes a range of angles in an optical system * nA, the symbol for nanoampere * Naturally aspirated engine Biology and medicine * Na (tree) or ''Mesua ferrea'', a species of tree native to Sri Lanka * Neuroacanthocytosis, a neurological condition * ''Nomina Anatomica'', a former international standard for human anatomical nomenclature * Noradrenaline, a hormone * Nucleic acid analogue, compounds analogous to naturally occurring RNA and DNA Places Current * Namibia (ISO country code) * Naples (car number plate code: NA), Italy * North America, a continent * North Africa, a subcontinent Historical * Netherlands Antilles (former international vehicle registration code: NA) * Na (Chinese state), a small state of the Chinese Zhou dynasty from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concentration Gradient
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately of dissolved salts (predominantly sodium () and chloride () ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at ) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about . The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was . Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measurement ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Dehydration
Osmotic dehydration is an operation used for the partial removal of water from plant tissues by immersion in a hypertonic (osmotic) solution. Sugar or salt solutions are used to reduce the moisture content of foods before actual drying process. This technique is used to give the product quality improvement over conventional drying process. Mild heat treatment after osmotic dehydration favours colour and flavour retention resulting in the product having superior organoleptic characteristics. It also increases resistance to heat treatment, prevents enzymatic browning and inhibits activities of polyphenol oxidase. The process is economical. Osmotic dehydration depends on: * Temperature of osmotic solution. * Concentration of the osmotic solution. * Osmotic agent used. * Process duration. * Geometry of food material. Process Water removal is based on the natural and non-destructive phenomenon of osmosis across cell membranes. The driving force for the diffusion of water from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)