|
Chiral Inversion
Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule. Chiral inversion happens depending on various factors (viz. biological-, solvent-, light-, temperature- induced, etc.) and the energy barrier associated with the stereogenic element present in the chiral molecule. 2-Arylpropionic acid nonsteroidal anti-inflammatory drugs (NSAIDs) provide one of the best pharmaceutical examples of chiral inversion. Chirality is attributed to a molecule due to the presence of a stereogenic element (viz. center, planar, helical, or axis). Many pharmaceutical drugs are chiral and have a labile (configurationally unstable) stereogenic element. Chiral compounds with stereogenic center are found to have high energy barriers for inversion and generally undergo biologically mediated chiral inversion. While compounds with helical or planar chirality have low energy barriers and chiral inversions are often cau ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, '' enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter how ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
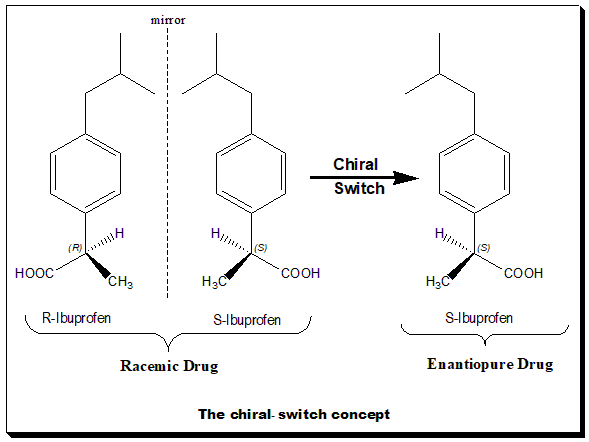
Chiral Switch
The word "chiral switch" was introduced by Agranat and Caner in 1999. Chiral switches are chiral drugs that are already approved as racemates but that have been re-developed as single enantiomers. The term chiral switching has been coined to describe the development of single enantiomers from racemate drugs. For example, levofloxacin is a chiral switch of racemic ofloxacin. The essential principle of a chiral switch is that there is a change in the status of chirality. In general, the term chiral switch is preferred over racemic switch because the switch is usually happening from a racemic drug to the corresponding single enantiomer(s). It is important to understand that chiral switches are treated as a selection invention. A selection invention is an invention that selects a group of new members from a previously known class on the basis of superior properties. To express the pharmacological activities of each of the chiral twins of a racemic drug two technical terms have been coi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dexibuprofen
Dexibuprofen is a nonsteroidal anti-inflammatory drug (NSAID). It is the active dextrorotatory enantiomer of ibuprofen. Most ibuprofen formulations contain a racemic mixture of both isomers. Chemistry Basically Dexibuprofen is a chiral switch of racemic ibuprofen. The chiral carbon in dexibuprofen is assigned an absolute configuration, (S), as per Cahn-Ingold-prelog rule. Pharmacology Ibuprofen, is an α-arylpropionic acid, used largely in the treatment of rheumatoid arthritis and widely used over-the counter drug for headache and minor pains. This drug has a chiral center and exists as a pair of enantiomers. The (S)- ibuprofen, the eutomer, is responsible for the desired therapeutic effect. Interestingly, the inactive (R)-enantiomer, the distomer, undergoes a unidirectional chiral inversion to give the active (S)-enantiomer, the former acting as a pro-drug for the latter. That is, when the ibuprofen is administered as a racemate the distomer is converted ''in vivo'' in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Chiral Inversion
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as ''catabolic'' – the ''breaking down'' of compounds (for example, of glucose to pyruvate by cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etoposide
Etoposide, sold under the brand name Vepesid among others, is a chemotherapy medication used for the treatments of a number of types of cancer including testicular cancer, lung cancer, lymphoma, leukemia, neuroblastoma, and ovarian cancer. It is also used for hemophagocytic lymphohistiocytosis. It is used by mouth or injection into a vein. Side effects are very common. They can include low blood cell counts, vomiting, loss of appetite, diarrhea, hair loss, and fever. Other severe side effects include allergic reactions and low blood pressure. Use during pregnancy will likely harm the fetus. Etoposide is in the topoisomerase inhibitor family of medication. It is believed to work by damaging DNA. Etoposide was approved for medical use in the United States in 1983. It is on the World Health Organization's List of Essential Medicines. Medical uses Etoposide is used as a form of chemotherapy for cancers such as Kaposi’s sarcoma, Ewing's sarcoma, lung cancer, testicular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pantoprazole
Pantoprazole, sold under the brand name Protonix, among others, is a proton pump inhibitor used for the treatment of stomach ulcers, short-term treatment of erosive esophagitis due to gastroesophageal reflux disease (GERD), maintenance of healing of erosive esophagitis, and pathological hypersecretory conditions including Zollinger–Ellison syndrome. It may also be used along with other medications to eliminate ''Helicobacter pylori''. Effectiveness is similar to other proton pump inhibitors (PPIs). It is available by mouth and by injection into a vein. Common side effects include headaches, diarrhea, vomiting, abdominal pain, and joint pain. More serious side effects may include severe allergic reactions, a type of chronic inflammation known as atrophic gastritis, ''Clostridium difficile'' colitis, low magnesium, and vitamin B12 deficiency. Use in pregnancy appears to be safe. Pantoprazole is a proton pump inhibitor that decreases gastric acid secretion. It works by inac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazolidinedione
The thiazolidinediones , abbreviated as TZD, also known as glitazones after the prototypical drug ciglitazone, are a class of heterocyclic compounds consisting of a five-membered C3NS ring. The term usually refers to a family of drugs used in the treatment of diabetes mellitus type 2 that were introduced in the late 1990s. Mechanism of action Thiazolidinediones or TZDs act by activating PPARs (peroxisome proliferator-activated receptors), a group of nuclear receptors, specific for ''PPARγ'' (PPAR-gamma, PPARG). They are thus the PPARG agonists subset of PPAR agonists. The endogenous ligands for these receptors are free fatty acids (FFAs) and eicosanoids. When activated, the receptor binds to DNA in complex with the retinoid X receptor (RXR), another nuclear receptor, increasing transcription of a number of specific genes and decreasing transcription of others. The main effect of expression and repression of specific genes is an increase in the storage of fatty acids in adip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albendazole
Albendazole (also known as albendazolum) is a broad-spectrum anthelmintic and antiprotozoal agent of the benzimidazole type. It is used for the treatment of a variety of intestinal parasite infections, including ascariasis, pinworm infection, hookworm infection, trichuriasis, strongyloidiasis, taeniasis, clonorchiasis, opisthorchiasis, cutaneous larva migrans, giardiasis, and gnathostomiasis, among other diseases. Common side effects include nausea, abdominal pain, and headache. Rare but potentially serious side effects include bone marrow suppression which usually improves on discontinuing the medication. Liver inflammation has been reported and those with prior liver problems are at greater risk. It is pregnancy category C in the United States and category D in Australia, meaning it may cause harm if taken by pregnant women. Albendazole was developed in 1975. It is on the World Health Organization's List of Essential Medicines. Medical uses Albendazole is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clopidogrel
Clopidogrel — sold under the brand name Plavix, among others — is an antiplatelet medication used to reduce the risk of heart disease and stroke in those at high risk. It is also used together with aspirin in heart attacks and following the placement of a coronary artery stent ( dual antiplatelet therapy). It is taken by mouth. Its effect starts about two hours after intake and lasts for five days. Common side effects include headache, nausea, easy bruising, itching, and heartburn. More severe side effects include bleeding and thrombotic thrombocytopenic purpura. While there is no evidence of harm from use during pregnancy, such use has not been well studied. Clopidogrel is in the thienopyridine-class of antiplatelets. It works by irreversibly inhibiting a receptor called P2Y12 on platelets. Clopidogrel was patented in 1982, and approved for medical use in 1997. It is on the World Health Organization's List of Essential Medicines. In 2020, it was the 29th most com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |