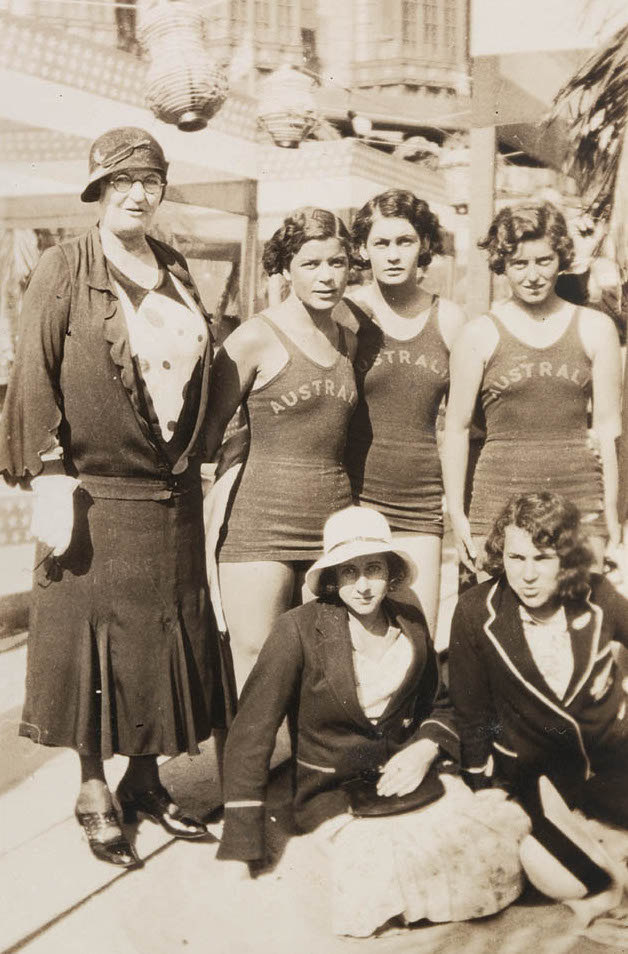|
Chaperone Therapy
Chaperone or Chaperon may refer to: *Chaperone (social) or chaperon, a person who accompanies or supervises young people on social occasions * Chaperone (clinical), a person who acts as a witness during a medical examination or procedure * Chaperon (headgear), a form of hood or hat worn in Western Europe in the Middle Ages Biology * Chaperone (protein), a protein that assists non-covalent folding/unfolding *Co-chaperone, a protein that assists a chaperone in protein folding and other functions *Pharmacological chaperone, a molecule which stabilizes protein folding, used in treatment for loss of function *Chaperone-mediated autophagy, a selective type of autophagy * MEAI or "Chaperon", a pharmaceutical drug used as a safer alternative to alcohol People * Bob Chaperon (born 1958), a retired Canadian snooker and billiards player * Nicolas Chaperon (died 1656), a French painter, draughtsman and engraver * Philippe Chaperon (1823–1907), a French scenographer See also *Chaperonin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone (social)
A chaperone (also spelled chaperon) in its original social usage was a person who for propriety's sake accompanied an unmarried girl in public; usually she was an older married woman, and most commonly the girl's own mother. In modern social usage, a chaperon (frequent in British spelling) or chaperone (usual in American spelling) is a responsible adult who accompanies and supervises young people. By extension, the word chaperone is used in clinical contexts. Origin The word derives figuratively from the French word ''chaperon'' (originally from the Late Latin ''cappa'', meaning "cape"), which referred to a hood that was worn by individuals generally. A chaperone was part of the costume of the Knights of the Garter when they were in full dress and, probably, since the Knights were court attendants, the word ''chaperon'' changed to mean escort. An alternative explanation comes from the sport of falconry, where the word meant the hood placed over the head of a bird of prey ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone (clinical)
In clinical medicine, a chaperone is a person who serves as a witness for both a patient and a medical practitioner as a safeguard for both parties during a medical examination or procedure. The exact responsibilities vary according to the clinical situation. Chaperones are widely used for gynecological and other intimate examination A well-woman examination is an exam offered to women to review elements of their reproductive health. The exam includes a breast examination, a pelvic examination and a Pap smear but may also include other procedures. Hospitals employ strict poli ...s. A chaperone may support the patient with reassurance and emotional support during a procedure or examination that the patient may find embarrassing or uncomfortable. The chaperone may also provide practical help to the doctor during an examination or procedure. In other clinical settings the chaperone could protect the doctor from physical attack. As a witness, the chaperone can help the doctor disp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperon (headgear)
A chaperon ( or ; Middle French: ''chaperon'') was a form of hood or, later, highly versatile hat worn in all parts of Western Europe in the Middle Ages. Initially a utilitarian garment, it first grew a long partly decorative tail behind called a liripipe, and then developed into a complex, versatile and expensive headgear after what was originally the vertical opening for the face began to be used as a horizontal opening for the head. It was especially fashionable in mid-15th century Burgundy, before gradually falling out of fashion in the late 15th century and returning to its utilitarian status. It is the most commonly worn male headgear in Early Netherlandish painting, but its complicated construction is often misunderstood. Humble origins The chaperon began before 1200 as a hood with a short cape, put on by pulling over the head, or fastening at the front. The hood could be pulled off the head to hang behind, leaving the short cape round the neck and shoulders. The edge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone (protein)
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding interme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Co-chaperone
Co-chaperones are proteins that assist chaperone (protein), chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulation of the ATPase activity of these chaperone proteins. There are a great number of different co-chaperones however based on their domain structure most of them fall into two groups: J-domain proteins and tetratricopeptide repeats (TPR). Co-chaperones assist heat shock proteins in the protein folding process. These co-chaperones can function in a number of ways. Primarily co-chaperones are involved in the ATPase functionality of their associated heat shock proteins. Co-chaperones catalyze the hydrolysis ATP to ADP on their respective chaperones which then allows them undergo a large conformational change that allows them to either bind to their substrates with higher affinity or aid in the release of the substrate followin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacological Chaperone
A pharmacological chaperone or pharmacoperone is a drug that acts as a protein chaperone. That is, it contains small molecules that enter cells and serve as a molecular scaffolding in order to cause otherwise- misfolded mutant proteins to fold and route correctly within the cell. Mutation of proteins often causes molecular misfolding, which results in protein misrouting within the cell. Accordingly, mutant molecules may retain proper function but end up in parts of the cell where the function is inappropriate, or even deleterious, to cell function. Misfolded proteins are usually recognized by the quality-control system of the cell and retained (and often destroyed or recycled) in the endoplasmic reticulum. Pharmacoperones correct the folding of misfolded proteins, allowing them to pass through the cell's quality-control system and become correctly routed. Since mutations often cause disease by causing misfolding and misrouting, pharmacoperones are potentially therapeutic agents, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone-mediated Autophagy
Chaperone-mediated autophagy (CMA) refers to the chaperone-dependent selection of soluble cytosolic proteins that are then targeted to lysosomes and directly translocated across the lysosome membrane for degradation. The unique features of this type of autophagy are the selectivity on the proteins that are degraded by this pathway and the direct shuttling of these proteins across the lysosomal membrane without the requirement for the formation of additional vesicles (Figure 1). Molecular components and steps The proteins that are degraded through CMA are cytosolic proteins or proteins from other compartments once they reach the cytosol. Therefore, some of the components that participate in CMA are present in the cytosol while others are located at the lysosomal membrane (Table I). Specific selection of proteins for degradation in all forms of autophagy came to further understanding as studies discovered the role of chaperones like hsc70. Although hsc70 targets cytosolic protein to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MEAI
MEAI (5-methoxy-2-aminoindane or 5-MeO-AI or Chaperon) belongs to the indane family of molecules. Its molecular structure was first mentioned implicitly in a markush structure schema appearing in a patent from 1998. It was later explicitly and pharmacologically described in a peer reviewed paper in 2017 by David Nutt and Ezekiel Golan et al. followed by another in February 2018 which detailed the pharmacokinetics, pharmacodynamics and metabolism of MEAI by Shimshoni, David Nutt, Ezekiel Golan et al. One year later it was studied and reported on in another peer reviewed paper by Halberstadt et al. The aminoindane family of molecules was, perhaps, first chemically described in 1980. MEAI was an early candidate of alcohol (drug), alcohol replacement drugs that came to market during a late 2010s movement to replace alcohol with less-toxic alternatives spearheaded by British Psychopharmacology, psychopharmacologist David Nutt rippling to the rest of Europe. In an act of gonzo journali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bob Chaperon
Robert Chaperon (born 18 May 1958) is a Canadians, Canadian retired professional snooker and billiards player. Career Chaperon was born on 18 May 1958. He played snooker on the professional tour from 1984 to 1995, and in the 1998/99, 2000/01, 2002/03 seasons, and also participated in the World Snooker Americas Tour in 1998/99, 1999/2000 and 2001/02. He won the 1990 British Open, beating Alex Higgins 10–8 in Higgins' last appearance in a major final. He reached one other ranking quarter-final, at the 1987 Grand Prix (snooker), 1987 Grand Prix. He also won the 1990 World Cup (snooker), 1990 World Cup as a member of the Canadian team, and the Canadian Snooker Championship in 1981, defeating Carey Lorraine in Ottawa. Having not played competitively for about three years, Chaperon resumed in 2007. In October 2019 he won a qualifier for the 2020 World Seniors Championship and although he was due to play in the event at the Crucible Theatre in August 2020, did not participate in the tou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicolas Chaperon
Nicolas Chaperon (bapt. 19 October 1612, in Châteaudun – 1656 in Lyon) was a French painter, draughtsman and engraver, a student in Paris of Simon Vouet whose style he adopted before he was further matured by his stay in Rome (1642–51) in the studio of Nicolas Poussin. In 1653-55 the consuls de Lyon called him to decorate the '' hôtel de ville'' but Chaperon dying almost as soon as he arrived, the commission passed to Thomas Blanchet. Chaperon made a name for himself with his suite of engravings after the Raphael Loggie of the Vatican, Rome, 1649, but art historians remember him for the stream of fulminating invective with which Poussin in his correspondence with Paul Fréart de Chantelou described this unruly and vindictive practician who refused to carry through his copy of a ''Transfiguration.'' So little is known of Chaperon that this episode stands out. Most of his paintings have been optimistically attributed to Poussin, and disguised under that sellable name have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Philippe Chaperon
Philippe Chaperon (2 February 1823 – 21 December 1906) was a French painter and scenic designer, particularly known for his work at the Paris Opera. He produced stage designs for the premieres of numerous 19th-century operas, including Verdi's ''Don Carlos'' and ''Aida'', Massenet's ''Le Cid'', Saint-Saëns's '' Henri VIII'', part two of Berlioz's ''Les Troyens'' and the first performances in France of Verdi's ''Otello'' and ''Rigoletto'' and Wagner's ''Tannhäuser''. Life and career Chaperon came from a modest background. He was born in Paris, where his father was an employee at the Caisse d'Épargne. He attended the Lycée impérial Bonaparte and then the École des Beaux-Arts where he studied painting and architecture. He won a Prix de Rome scholarship and spent three years at the Villa Medici. He also studied architecture in the atelier of Victor Baltard and painting in the atelier of Léon Riesener where he received guidance from Riesener's cousin Eugène Delacroix. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperonin
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional tertiary structure. The energy to fold proteins is supplied by non-covalent interactions between the amino acid side chains of each protein, and by solvent effects. Most proteins spontaneously fold into their most stable three-dimensional conformation, which is usually also their functional conformation, but occasionally proteins mis-fold. Molecular chaperones catalyze protein refolding by accelerating partial unfolding of misfolded proteins, aided by energy supplied by the hydrolysis of adenosine triphosphate (ATP). Chaperonin proteins may also tag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |