|
Cathomycin
Novobiocin, also known as albamycin or cathomycin, is an aminocoumarin antibiotic that is produced by the actinomycete ''Streptomyces niveus'', which has recently been identified as a subjective synonym for ''S. spheroides'' a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1.Alessandra da Silva Eustáquio (2004) Biosynthesis of aminocoumarin antibiotics in Streptomyces: Generation of structural analogues by genetic engineering and insights into the regulation of antibiotic production. DISSERTATION Novobiocin was first reported in the mid-1950s (then called streptonivicin). Clinical use It is active against ''Staphylococcus epidermidis'' and may be used to differentiate it from the other coagulase-negative ''Staphylococcus saprophyticus'', which is resistant to novobiocin, in culture. Novobiocin was licensed for clinical use under the tradename Albamycin (Upjohn) in the 1960s. Its efficacy has been demonstrated in preclinic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
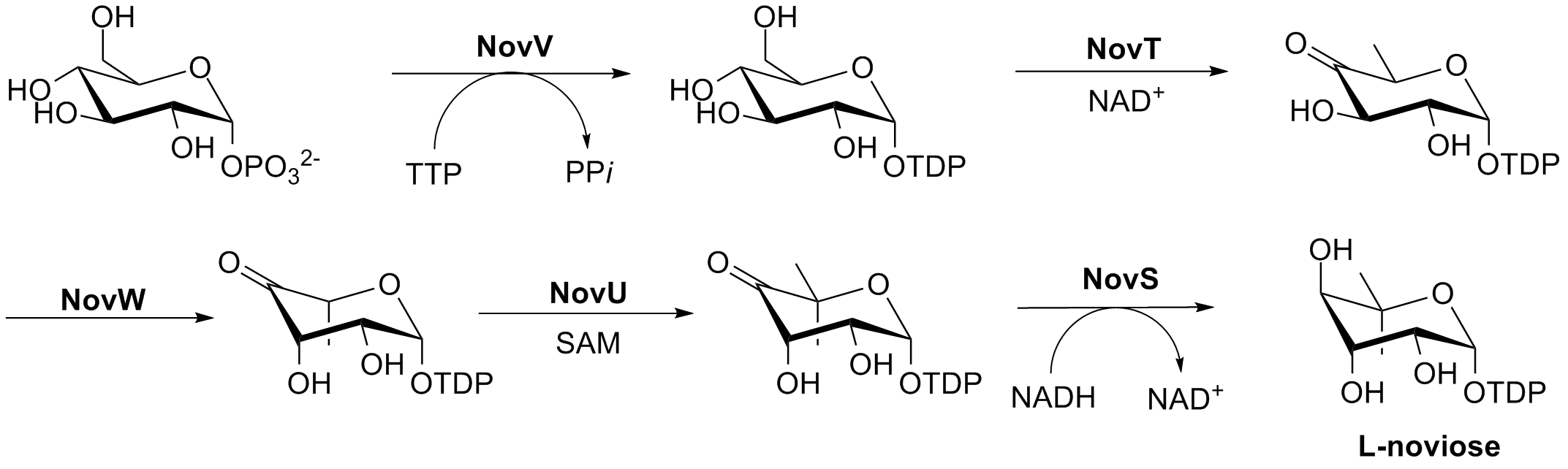
Novobiocin Ring B Synthesis
Novobiocin, also known as albamycin or cathomycin, is an aminocoumarin antibiotic that is produced by the actinomycete ''Streptomyces niveus'', which has recently been identified as a subjective synonym for ''S. spheroides'' a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1.Alessandra da Silva Eustáquio (2004) Biosynthesis of aminocoumarin antibiotics in Streptomyces: Generation of structural analogues by genetic engineering and insights into the regulation of antibiotic production. DISSERTATION Novobiocin was first reported in the mid-1950s (then called streptonivicin). Clinical use It is active against ''Staphylococcus epidermidis'' and may be used to differentiate it from the other coagulase-negative ''Staphylococcus saprophyticus'', which is resistant to novobiocin, in culture. Novobiocin was licensed for clinical use under the tradename Albamycin (Upjohn) in the 1960s. Its efficacy#Pharmacology, efficacy has been de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albomycin
Albomycins are a group of naturally occurring antibiotics belonging to the class of sideromycins, which are "compounds composed of iron carriers called siderophores linked to antibiotic moieties". They are particularly effective against Gram-negative bacteria of the family '' Enterobacteriaceae'' and few Gram-positive bacteria such s ''Streptococcus pneumoniae'', ''Bacillus subtilis'' and '' Staphylococcus aureus''. In 2000 a group of scientists from SmithKline Beecham Pharmaceuticals, UK reported that the antibiotic part of albomycin ''in vitro'' can inhibit seryl t-RNA synthetase from both eukaryotic and prokaryotic representatives. Structure Albomycins are naturally occurring sideromycins produced by some streptomycetes. The siderophore part of albomycin δ2 is similar to ferrichrome. It contains three molecules of δ-N-hydroxy-δ-N-acetyl ornithine linked to a serine, all by peptide linkage. The C-terminus of the serine is linked to another serine attached to the antibi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
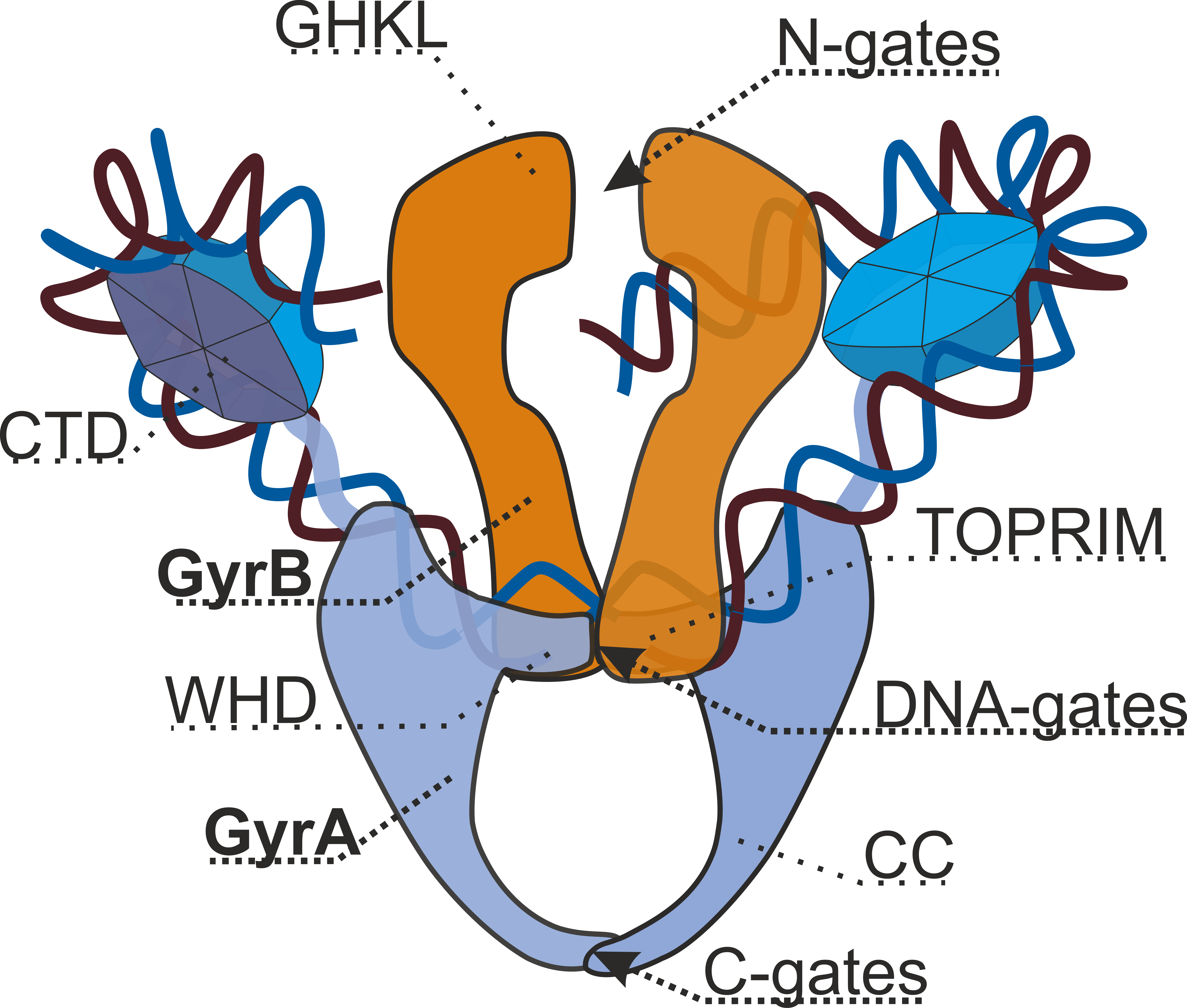
DNA Gyrase
DNA gyrase, or simply gyrase, is an enzyme Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ... within the class of topoisomerase and is a subclass of Type II topoisomerases that reduces topological strain in an ATP dependent manner while double-stranded DNA is being unwound by elongating RNA polymerase, RNA-polymerase or by helicase in front of the progressing DNA replication#Replication fork, replication fork. The enzyme causes negative DNA supercoil, supercoiling of the DNA or relaxes positive supercoils. It does so by looping the template so as to form a crossing, then cutting one of the double helices and passing the other through it before releasing the break, changing the linking number by two in each enzymatic step. This process occurs in bacteria, whose single circular DNA is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The cofactor is, therefore, found in two forms in cells: NAD is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
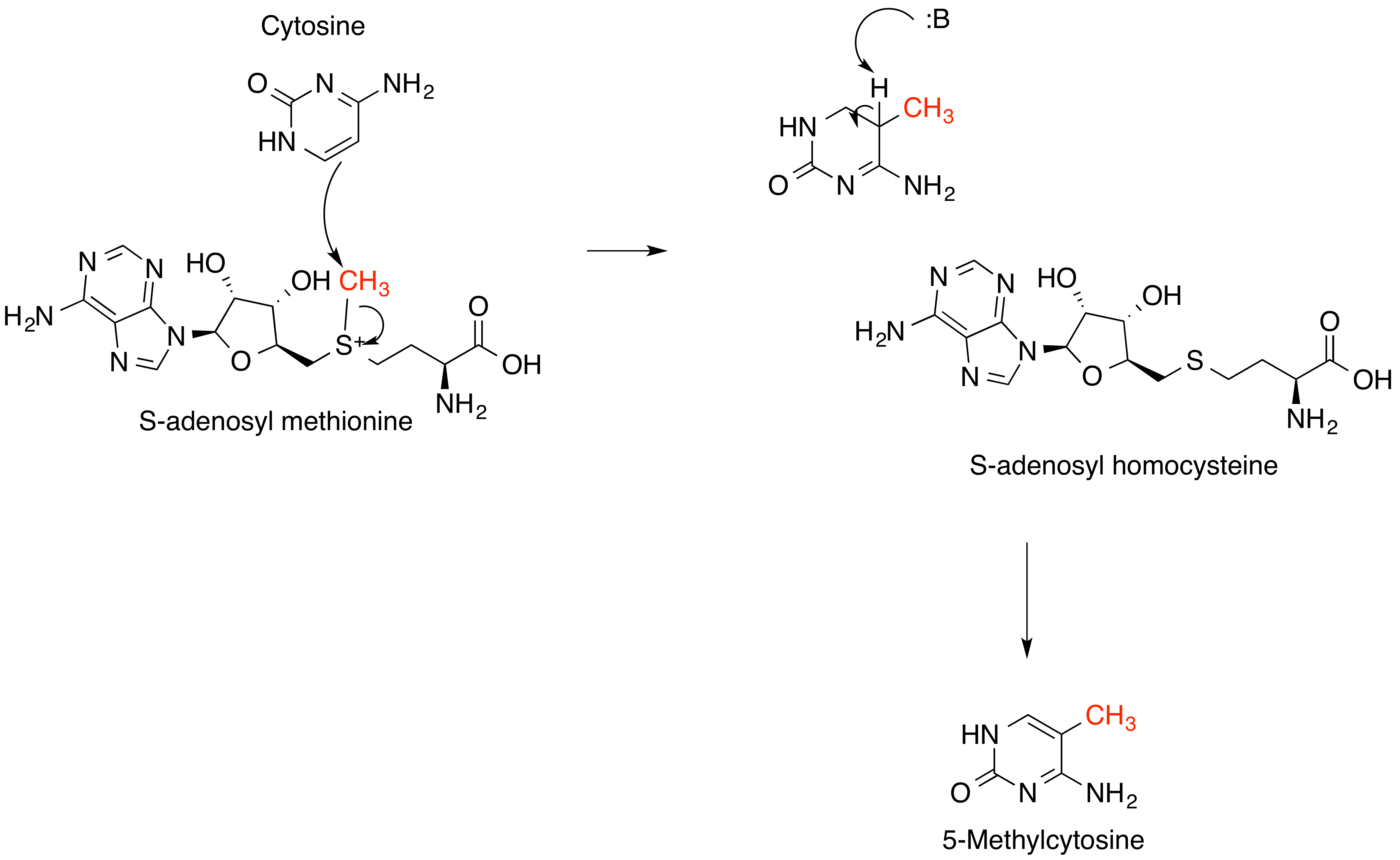
S-adenosyl Methionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme. From the perspective of biochemistry, ATP is classified as a nucleoside triphosphate, which indicates that it consists of three components: a nitrogenous base (adenine), the sugar ribose, and the Polyphosphate, triphosphate. Structure ATP consists of an adenine attached by the 9-nitrogen atom to the 1′ carbon atom of a sugar (ribose), which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning ''cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a Hydrophobe, hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is Genetic code, encoded by the Genetic code#Codons, codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mandler NovoRevisions
Mandler is a surname. Notable people with the surname include: *Albert Mandler (1929–1973), Israeli general *Anthony Mandler (born 1973), American music video director *George Mandler (1924–2016), American psychologist * James Mandler (1922–2007), American basketball player *Jean Matter Mandler Jean Matter Mandler (born 1929) is Distinguished Research Professor of Cognitive Science at the University of California, San Diego and visiting professor at University College London. She was born in Oak Park, Illinois, in 1929 and attended Ca ... (born 1929), American psychologist * Peter Mandler (born 1958), British historian and academic * Walter Mandler (1922–2005), lens designer {{surname, Mandler ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life. NADPH is the reduced form of NADP. NADP differs from NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, and NADP+ phosphatase can convert NADPH back to NADH to maintain a balance. Some forms of the NAD+ kinas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shikimic Acid
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower ''shikimi'' (, the Japanese star anise, ''Illicium anisatum''), from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later. Biosynthesis Phosphoenolpyruvate and erythrose-4-phosphate condense to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. DAHP is then transformed to 3-dehydroquinate (DHQ), in a reaction catalyzed by DHQ synthase. Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD. : DHQ is dehydrated to 3-dehydroshikimic acid by the enzyme 3-dehydroquinate dehydratase, which is reduced to shiki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylallyl Pyrophosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP. In the mevalonate pathway DMAPP is synthesised from mevalonic acid. In contrast, DMAPP is synthesised from HMBPP in the MEP pathway. At present, it is believed that there is crossover between the two pathways in organisms that use both pathways to create terpenes and terpenoid The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes" ...s, such as in plants, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prephenate
Prephenic acid, commonly also known by its anionic form prephenate, is an intermediate in the biosynthesis of the aromatic amino acids phenylalanine and tyrosine, as well as of a large number of secondary metabolites of the shikimate pathway. It is biosynthesized by a ,3sigmatropic Claisen rearrangement of chorismate. : Stereochemistry Prephenic acid is an example of achiral (optically inactive) molecule which has two pseudoasymmetric atoms (''i.e.'' stereogenic but not chirotopic centers), the C1 and the C4 cyclohexadiene Cyclohexadiene may refer to: * 1,3-Cyclohexadiene, * 1,4-Cyclohexadiene, See also * Benzene or its theoretical isomer ''1,3,5-Cyclohexatriene'' * Cyclohexene Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorle ... ring atoms. It has been shown that of the two possible diastereoisomers, the natural prephenic acid is one that has both substituents at higher priority (according to CIP rules) on the two pseudoasymmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |