|
Castanospermine
Castanospermine is an indolizidine alkaloid first isolated from the seeds of ''Castanospermum australe''. It is a potent inhibitor of some glucosidase enzymes and has antiviral activity ''in vitro'' and in mouse models. The castanospermine derivative celgosivir is an antiviral drug candidate currently in development for possible use in treating hepatitis C virus (HCV) infection. Biosynthesis of castanospermine L-Lys undergoes a transamination to form α-aminoadipic acid. α-aminoadipic acid undergoes a ring closure and then a reduction to form L-pipecolic acid (Figure 1). In the alternate pathway (Figure 2), L-Lys cyclizes and forms the enamine, which reduces to L-pipecolic acid. HSCoA and then malonyl-CoA react in a Claisen reaction with L-pipecolic acid to form SCoA ester which undergoes a ring closure to form 1-indolizidinone. The carbonyl on 1-indolizidinone is reduced to the hydroxyl group. The molecule is then further hydroxylated to form the final product castanospermin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Castanospermine Biosynthesis With Enamine Intermediate
Castanospermine is an indolizidine alkaloid first isolated from the seeds of ''Castanospermum australe''. It is a potent inhibitor of some glucosidase enzymes and has antiviral activity ''in vitro'' and in mouse models. The castanospermine derivative celgosivir is an antiviral drug candidate currently in development for possible use in treating hepatitis C virus (HCV) infection. Biosynthesis of castanospermine L-Lys undergoes a transamination to form α-aminoadipic acid. α-aminoadipic acid undergoes a ring closure and then a reduction to form L-pipecolic acid (Figure 1). In the alternate pathway (Figure 2), L-Lys cyclizes and forms the enamine, which reduces to L-pipecolic acid. HSCoA and then malonyl-CoA react in a Claisen reaction with L-pipecolic acid to form SCoA ester which undergoes a ring closure to form 1-indolizidinone. The carbonyl on 1-indolizidinone is reduced to the hydroxyl group. The molecule is then further hydroxylated to form the final product castanospermine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Castanospermum Australe
''Castanospermum australe'' (Moreton Bay chestnut or blackbean), the only species in the genus ''Castanospermum'', is a flowering plant in the family Fabaceae, native to the east coast of Australia in Queensland and New South Wales, and to the Pacific islands of Vanuatu, New Caledonia, and the island of New Britain (Papua New Guinea). Growth It is a large evergreen tree growing to tall, though commonly much smaller. The leaves are long and broad, pinnate, with 11-15 leaflets. The flowers are bicoloured red and yellow, long, produced in racemes long. The fruit is a cylindrical pod long and diameter, the interior divided by a spongy substance into one to five cells, each of which contains a large chestnut-like seed. Common names The 1889 book 'The Useful Native Plants of Australia' records the common names of ''Castanospermum australe'' as "Moreton Bay Chestnut" and "Bean tree" and notes that it was called "Irtalie" by Aboriginal people of the Richmond and Clarence Rivers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Swainsonine
Swainsonine is an indolizidine alkaloid. It is a potent inhibitor of Golgi alpha-mannosidase II, an immunomodulator, and a potential chemotherapy drug. As a toxin in locoweed (likely its primary toxin) it also is a significant cause of economic losses in livestock industries, particularly in North America. It was first isolated from '' Swainsona canescens''. Pharmacology Swainsonine inhibits glycoside hydrolases, specifically N-linked glycosylation. Disruption of Golgi alpha-mannosidase II with swainsonine induces hybrid-type glycans. These glycans have a Man5GlcNAc2 core with processing on the 3-arm that resembles so-called complex-type glycans. The pharmacological properties of this product have not been fully investigated. Sources Some plants do not produce the toxic compound itself; they are host of endophytic fungi which produces swainsonine. Biosynthesis The biosynthesis of swainsonine has been investigated in the fungus ''Rhizoctonia leguminicola'', and it i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Merck Index
''The Merck Index'' is an encyclopedia of chemicals, drugs and biologicals with over 10,000 monograph on single substances or groups of related compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an appendix with monographs on organic named reactions. The 15th edition was published in A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Alkaloids Found In Fabaceae
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , |
 |
Alkaloids
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including , , |
|
Alternate Pathway For The Formation Of Pipecolic Acid In The Biosynthesis Of Castanospermine
Alternative or alternate may refer to: Arts, entertainment and media * Alternative (''Kamen Rider''), a character in the Japanese TV series ''Kamen Rider Ryuki'' * ''The Alternative'' (film), a 1978 Australian television film * ''The Alternative'', a radio show hosted by Tony Evans * ''120 Minutes'' (2004 TV program), an alternative rock music video program formerly known as ''The Alternative'' *''The American Spectator'', an American magazine formerly known as ''The Alternative: An American Spectator'' * Alternative comedy, a range of styles used by comedians and writers in the 1980s * Alternative comics, a genre of comic strips and books * Alternative media, media practices falling outside the mainstreams of corporate communication * Alternative reality, in fiction * Alternative title, the use of a secondary title for a work when it is distributed or sold in other countries Music * ''Alternative'' (album), a B-sides album by Pet Shop Boys * ''The Alternative'' (album), an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Celgosivir
Celgosivir, in development by Migenix for the treatment of hepatitis C virus (HCV) infection, is an oral prodrug of the natural product castanospermine that inhibits alpha-glucosidase I, an enzyme that plays a critical role in viral maturation by initiating the processing of the N-linked oligosaccharides of viral envelope glycoproteins. Celgosivir is well absorbed ''in vitro'' and ''in vivo'', and is rapidly converted to castanospermine. Celgosivir has a novel mechanism of action (preventing the glycosylation of viral proteins by the host), and demonstrates broad antiviral activity ''in vitro''. Clinical trials Celgosivir is not efficient as a monotherapy for the treatment of HCV, but has demonstrated a synergistic effect in combination with pegylated interferon alfa-2b plus ribavirin, both ''in vitro'' and in phase II clinical trials that last up to 1 year in patients with chronic HCV infection. Celgosivir may prove to be a valuable component for combination therapy and may help ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
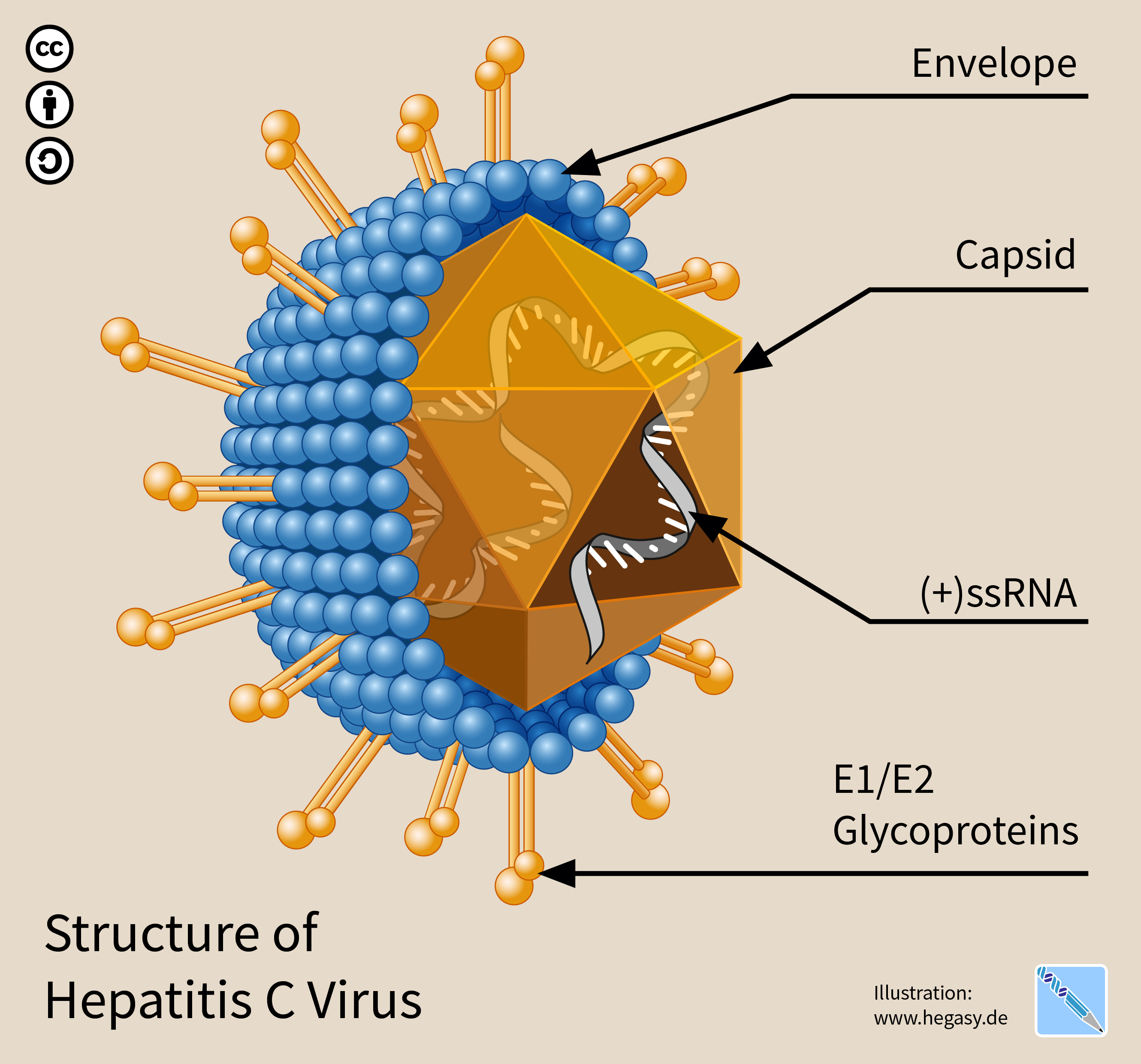 |
Hepatitis C Virus
The hepatitis C virus (HCV) is a small (55–65 nm in size), enveloped, positive-sense single-stranded RNA virus of the family '' Flaviviridae''. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer (hepatocellular carcinoma, abbreviated HCC) and lymphomas in humans. Taxonomy The hepatitis C virus belongs to the genus '' Hepacivirus'', a member of the family '' Flaviviridae''. Before 2011, it was considered to be the only member of this genus. However a member of this genus has been discovered in dogs: canine hepacivirus. There is also at least one virus in this genus that infects horses. Several additional viruses in the genus have been described in bats and rodents. Structure The hepatitis C virus particle consists of a lipid membrane envelope that is 55 to 65 nm in diameter. Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope. They take part in viral attachment and entry into the cell. Within the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Indolizidine
Indolizidine is a heterocyclic chemical compound that forms the central core of the indolizidine alkaloids such as swainsonine and castanospermine. See also * Indole * Indolizine * Tryptophan * Tryptamine Tryptamine is an indolamine metabolite of the essential amino acid, tryptophan. The chemical structure is defined by an indole ─ a fused benzene and pyrrole ring, and a 2-aminoethyl group at the second carbon (third aromatic atom, with the f ... References Indolizidines {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is avai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |