|
Carrier Plasma Glycoprotein
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated. In proteins that have segments extending extracellularly, the extracellular segments are also often glycosylated. Glycoproteins are also often important integral membrane proteins, where they play a role in cell–cell interactions. It is important to distinguish endoplasmic reticulum-based glycosylation of the secretory system from reversible cytosolic-nuclear glycosylation. Glycoproteins of the cytosol and nucleus can be modified through the reversible addition of a single GlcNAc residue that is considered reciprocal to phosphorylation and the functions of these are likely to be an additional regulatory mechanism that controls phosphorylation-based signalling. In contrast, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tryptophan
Tryptophan (symbol Trp or W) is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α-carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic beta carbon substituent. It is essential in humans, meaning that the body cannot synthesize it and it must be obtained from the diet. Tryptophan is also a precursor to the neurotransmitter serotonin, the hormone melatonin, and vitamin B3. It is encoded by the codon UGG. Like other amino acids, tryptophan is a zwitterion at physiological pH where the amino group is protonated (–; pKa = 9.39) and the carboxylic acid is deprotonated ( –COO−; pKa = 2.38). Humans and many animals cannot synthesize tryptophan: they need to obtain it through their diet, making it an essential amino acid. Function Amino acids, including tryptophan, are used as building blocks in protein biosynthesis, and proteins are required to sustain life. Man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mannose
Mannose is a sugar monomer of the aldohexose series of carbohydrates. It is a C-2 epimer of glucose. Mannose is important in human metabolism, especially in the glycosylation of certain proteins. Several congenital disorders of glycosylation are associated with mutations in enzymes involved in mannose metabolism. Mannose is not an essential nutrient; it can be produced in the human body from glucose, or converted into glucose. Mannose provides 2–5 kcal/g. It is partially excreted in the urine. Etymology The root of both "mannose" and "mannitol" is manna, which the Bible describes as the food supplied to the Israelites during their journey in the region of Sinai. Several trees and shrubs can produce a substance called manna, such as the "manna tree" ('' Fraxinus ornus'') from whose secretions mannitol was originally isolated. Structure Mannose commonly exists as two different-sized rings, the pyranose (six-membered) form and the furanose (five-membered) form. E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoserine
Phosphoserine (abbreviated as SEP or J) is an ester of serine and phosphoric acid. Phosphoserine is a component of many proteins as the result of posttranslational modifications. The phosphorylation of the alcohol functional group in serine to produce phosphoserine is catalyzed by various types of kinases. Through the use of technologies that utilize an expanded genetic code, phosphoserine can also be incorporated into proteins during translation. It is a normal metabolite In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, ... found in human biofluids. Phosphoserine has three potential coordination sites (carboxyl, amine and phosphate group) Determination of the mode of coordination between phosphorylated ligands and metal ions occurring in an organism is a first step to explain the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorous Acid
Phosphorous acid (or phosphonic acid (singular)) is the compound described by the formula H3PO3. This acid is diprotic (readily ionizes two protons), not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids. Nomenclature and tautomerism H3PO3 is more clearly described with the structural formula HPO(OH)2. In the solid state, has tetrahedral geometry about the central phosphorus atom, with a P–H bond of 132 pm, one P=O double bond of 148 pm and two longer P–O(H) single bonds of 154 pm. This species exists in equilibrium with an extremely minor tautomer . IUPAC recommends that the latter be called phosphorous acid, whereas the dihydroxy form is called phosphonic acid.. Only the reduced phosphorus compounds are spelled with an "ous" ending. Other important oxyacids of ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
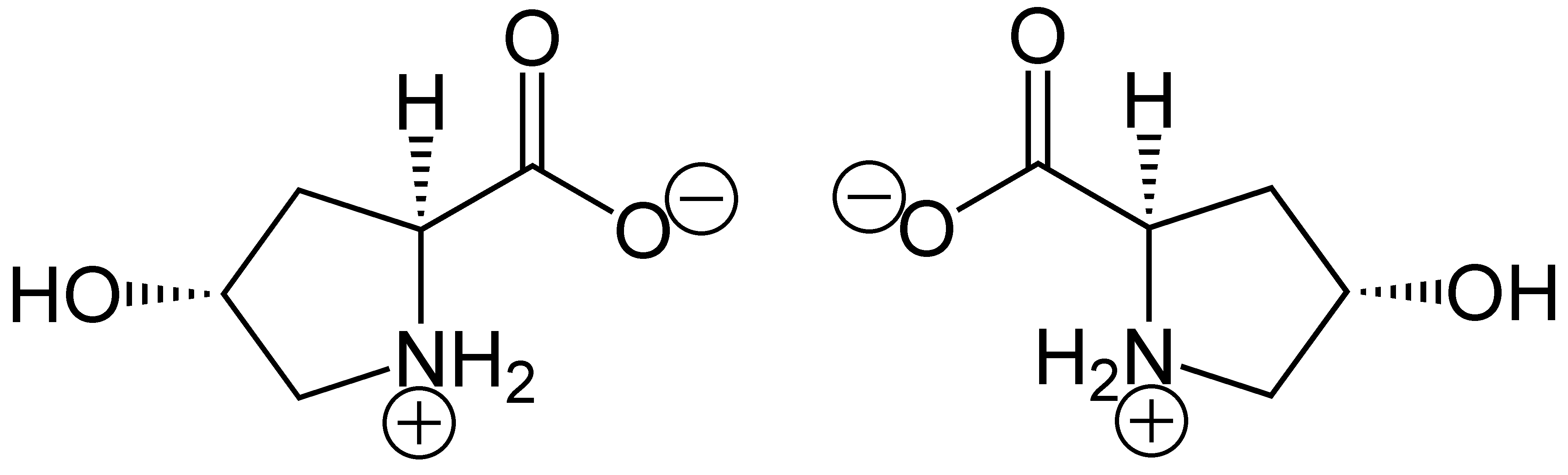
Hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank. Structure and discovery In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom. Production and function Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl hydroxylase following protein synthesis (as a post-translational modification). The enzyme catalyzed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated. Animals Collagen Hydroxyproline is a major ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylysine
Hydroxylysine (Hyl) is an amino acid with the molecular formula C6H14N2O3. It was first discovered in 1921 by Donald Van Slyke Donald Dexter Van Slyke (March 29, 1883 – May 4, 1971) was a Dutch American biochemist. His achievements included the publication of 317 journal articles and 5 books, as well as numerous awards, among them the National Medal of Science and t ... as the 5-hydroxylysine form. It arises from a post-translational hydroxy modification of lysine. It is most widely known as a component of collagen. It is biosynthesized from lysine via oxidation by lysyl hydroxylase enzymes. The most common form is the (5''R'') stereoisomer found in collagen. However, the enzyme JMJD6 has recently been shown to be a lysyl hydroxylase which modifies an RNA splicing factor producing the (5''S'') stereoisomer. Additionally, in ''E. coli'', there has been at least one lysine ''N''-hydroxylase enzyme identified, named IucD. References External links * {{MeshName, Hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Greek ''tyrós'', meaning '' cheese'', as it was first discovered in 1846 by German chemist Justus von Liebig in the protein casein from cheese. It is called tyrosyl when referred to as a functional group or side chain. While tyrosine is generally classified as a hydrophobic amino acid, it is more hydrophilic than phenylalanine. It is encoded by the codons UAC and UAU in messenger RNA. Functions Aside from being a proteinogenic amino acid, tyrosine has a special role by virtue of the phenol functionality. It occurs in proteins that are part of signal transduction processes and functions as a receiver of phosphate groups that are transferred by way of protein kinases. Phosphorylation of the hydroxyl group can change the activity of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can underg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |