|
Calcium Pump
Calcium pumps are a family of ion transporters found in the cell membrane of all animal cells. They are responsible for the active transport of calcium out of the cell for the maintenance of the steep Ca2+ electrochemical gradient across the cell membrane. Calcium pumps play a crucial role in proper cell signalling by keeping the intracellular calcium concentration roughly 10,000 times lower than the extracellular concentration. Failure to do so is one cause of muscle cramps. The plasma membrane Ca2+ ATPase and the sodium-calcium exchanger are together the main regulators of intracellular Ca2+ concentrations. Biological role Ca2+ has many important roles as an intracellular messenger. The release of a large amount of free Ca2+ can trigger a fertilized egg to develop, skeletal muscle cells to contract, secretion by secretory cells and interactions with Ca2+ -responsive proteins like calmodulin. To maintain low concentrations of free Ca2+ in the cytosol, cells use membran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Transporter
In biology, a transporter is a transmembrane protein that moves ions (or other small molecules) across a biological membrane to accomplish many different biological functions including, cellular communication, maintaining homeostasis, energy production, etc. There are different types of transporters including, pumps, uniporters, antiporters, and symporters. Active transporters or ion pumps are transporters that convert energy from various sources—including adenosine triphosphate (ATP), sunlight, and other redox reactions—to potential energy by pumping an ion up its concentration gradient. This potential energy could then be used by secondary transporters, including ion carriers and ion channels, to drive vital cellular processes, such as ATP synthesis. This page is focused mainly on ion transporters acting as pumps, but transporters can also function to move molecules through facilitated diffusion. Facilitated diffusion does not require ATP and allows molecules, that are unable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
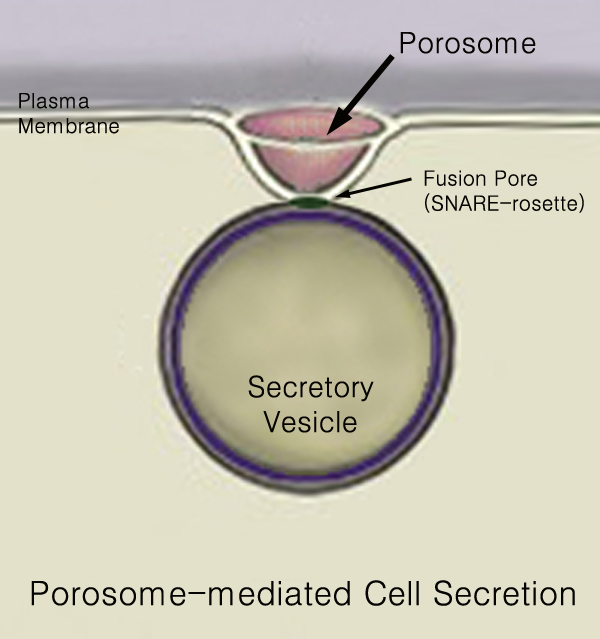
Secretion
440px Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. Secretion in bacterial species means the transport or translocation of effector molecules for example: proteins, enzymes or toxins (such as cholera toxin in pathogenic bacteria e.g. ''Vibrio cholerae'') from across the interior (cytoplasm or cytosol) of a bacterial cell to its exterior. Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment for adaptation and survival. In eukaryotic cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Biology
Cell biology (also cellular biology or cytology) is a branch of biology that studies the structure, function, and behavior of cells. All living organisms are made of cells. A cell is the basic unit of life that is responsible for the living and functioning of organisms. Cell biology is the study of structural and functional units of cells. Cell biology encompasses both prokaryotic and eukaryotic cells and has many subtopics which may include the study of cell metabolism, cell communication, cell cycle, biochemistry, and cell composition. The study of cells is performed using several microscopy techniques, cell culture, and cell fractionation. These have allowed for and are currently being used for discoveries and research pertaining to how cells function, ultimately giving insight into understanding larger organisms. Knowing the components of cells and how cells work is fundamental to all biological sciences while also being essential for research in biomedical fields such as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Site
In biochemistry and molecular biology, a binding site is a region on a macromolecule such as a protein that binds to another molecule with specificity. The binding partner of the macromolecule is often referred to as a ligand. Ligands may include other proteins (resulting in a protein-protein interaction), enzyme substrates, second messengers, hormones, or allosteric modulators. The binding event is often, but not always, accompanied by a conformational change that alters the protein's function. Binding to protein binding sites is most often reversible (transient and non-covalent), but can also be covalent reversible or irreversible. Function Binding of a ligand to a binding site on protein often triggers a change in conformation in the protein and results in altered cellular function. Hence binding site on protein are critical parts of signal transduction pathways. Types of ligands include neurotransmitters, toxins, neuropeptides, and steroid hormones. Binding sites in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Mass Unit
The dalton or unified atomic mass unit (symbols: Da or u) is a non-SI unit of mass widely used in physics and chemistry. It is defined as of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. The atomic mass constant, denoted ''m''u, is defined identically, giving . This unit is commonly used in physics and chemistry to express the mass of atomic-scale objects, such as atoms, molecules, and elementary particles, both for discrete instances and multiple types of ensemble averages. For example, an atom of helium-4 has a mass of . This is an intrinsic property of the isotope and all helium-4 atoms have the same mass. Acetylsalicylic acid (aspirin), , has an average mass of approximately . However, there are no acetylsalicylic acid molecules with this mass. The two most common masses of individual acetylsalicylic acid molecules are , having the most common isotopes, and , in which one carbon is carbon-13. The molecular mass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Transport
In cellular biology, ''active transport'' is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate ( ATP), and secondary active transport that uses an electrochemical gradient. Some examples of active transport include: * Phagocytosis of bacteria by macrophages * Movement of calcium ions out of cardiac muscle cells * Transportation of amino acids across the intestinal lining in the human gut * Secretion of proteins such as enzymes, peptide hormones, and antibodies from various cells * Functioning of white blood cells to defend invading diseases Active cellular transportation (ACT) Unlike passive transport, which uses the kinetic energy and natural entropy of molecules moving down a gradient, active ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sarcoplasmic Reticulum
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other Cell (biology), cells. The main function of the SR is to store calcium ions (Ca2+). Calcium in biology, Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondrion, mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
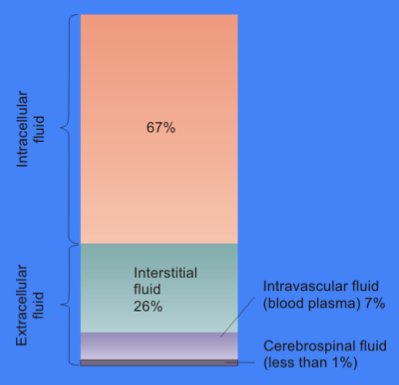
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and prope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases. Structure Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 kDa). The protein has two approximately symmetrical globular domains (the N- and C- domains) each containing a pair of EF hand motifs separated by a flexible linker region for a total of four Ca2+ binding sites, two in each globular domain. In the Ca2+-free state, the helices that form the four EF-hands are collapsed in a compact orientation, and the central linker is disordered; in the Ca2+-saturated state, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skeletal Muscle
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of muscle tissue, and are often known as muscle fibers. The muscle tissue of a skeletal muscle is striated – having a striped appearance due to the arrangement of the sarcomeres. Skeletal muscles are voluntary muscles under the control of the somatic nervous system. The other types of muscle are cardiac muscle which is also striated and smooth muscle which is non-striated; both of these types of muscle tissue are classified as involuntary, or, under the control of the autonomic nervous system. A skeletal muscle contains multiple muscle fascicle, fascicles – bundles of muscle fibers. Each individual fiber, and each muscle is surrounded by a type of connective tissue layer of fascia. Muscle fibers are formed from the cell fusion, fusion of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |