|
Butterfly Cluster Compound
In the area of metal cluster chemistry, a butterfly cluster compound usually describes tetrametallic clusters containing five M-M bonds. A prototype of this motif is e4(CO)16sup>2−. Most butterfly clusters have additional bridging ligand In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...s. One example is the penta phosphide Rh4(CO)5(PPh2)5− where all Rh---Rh edges are bridged by PPh2. A carbide-containing butterfly cluster is e4C(CO)12sup>2− where the carbide is bonded to all four Fe centers.{{cite journal, authors=Sappa, E.; Tiripicchio, A.; Carty, A. J.; Toogood, G. E., title=Butterfly Cluster Complexes of the Group VIII Transition Metals, journal=Progress in Inorganic Chemistry, year=1987, volume=35, page=437, doi=10.1002/9780470166369.ch5, isbn=9780470166369 Bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Cluster
In chemistry, an atom cluster (or simply cluster) is an ensemble of bound atoms or molecules that is intermediate in size between a simple molecule and a nanoparticle; that is, up to a few nanometers (nm) in diameter. The term ''microcluster'' may be used for ensembles with up to couple dozen atoms. Clusters with a definite number and type of atoms in a specific arrangement are often considered a specific chemical compound and are studied as such. For example, fullerene is a cluster of 60 carbon atoms arranged as the vertices of a truncated icosahedron, and decaborane is a cluster of 10 boron atoms forming an incomplete icosahedron, surrounded by 14 hydrogen atoms. The term is most commonly used for ensembles consisting of several atoms of the same element, or of a few different elements, bonded in a three-dimensional arrangement. Transition metals and main group elements form especially robust clusters. Indeed, in some contexts, the term may refer specifically to a metal cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
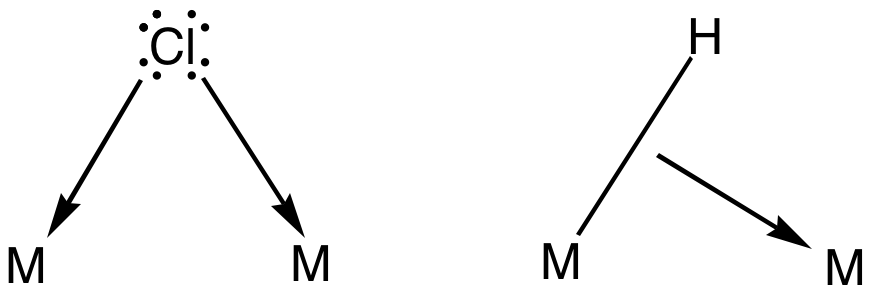
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphide
In chemistry, a phosphide is a compound containing the ion or its equivalent. Many different phosphides are known, with widely differing structures. Most commonly encountered on the binary phosphides, i.e. those materials consisting only of phosphorus and a less electronegative element. Numerous are polyphosphides, which are solids consisting of anionic chains or clusters of phosphorus. Phosphides are known with the majority of less electronegative elements with the exception of Hg, Pb, Sb, Bi, Te, and Po.Von Schnering, H.G. and Hönle , W. (1994) "Phosphides - Solid-state Chemistry" in ''Encyclopedia of Inorganic Chemistry''. R. Bruce King (ed.). John Wiley & Sons Finally, some phosphides are molecular. Binary phosphides Binary phosphides include phosphorus and one other element. An example of a group 1 phosphide is sodium phosphide (). Other notable examples include aluminium phosphide () and calcium phosphide (), which are used as pesticides, exploiting their tendenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbide Cluster
A metal carbido complex is a coordination complex that contains a carbon atom as a ligand. Carbido complexes are a molecular subclass of carbides, which are prevalent. Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis and related catalytic processes. They are also used as precursors for the synthesis of more complicated carbides. They are analogous to metal nitrido complexes. Carbido clusters Most molecular carbido complexes are clusters, usually featuring carbide as a six-fold bridging ligand. Examples include , and . The iron carbonyl carbides exist not only in the encapsulated carbon () but also with exposed carbon centres as in and . Clusters without CO ligands are also known. Doubly bridging carbide ligands Bridging carbido ligands can be subdivided into three classes: *cumulenic , *metallocarbyne , and *polar covalent . Cumulenic compounds generally bridge two metal atoms of the same element and are symmetrical. However, there are excep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force and hydrogen bonding. Strong chemical bonding arises from the sharing or transfer of electrons between the participating atoms. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyhedral Skeletal Electron Pair Theory
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such as borane and carborane clusters. The electron counting rules were originally formulated by Kenneth Wade, and were further developed by others including Michael Mingos; they are sometimes known as Wade's rules or the Wade–Mingos rules. The rules are based on a molecular orbital treatment of the bonding. These notes contained original material that served as the basis of the sections on the 4''n'', 5''n'', and 6''n'' rules. These rules have been extended and unified in the form of the Jemmis ''mno'' rules. Predicting structures of cluster compounds Different rules (4''n'', 5''n'', or 6''n'') are invoked depending on the number of electrons per vertex. The 4''n'' rules are reasonably accurate in predicting the structures of clusters having about 4 electrons per vertex, as is the case for many boranes and carboranes. For such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
15.png)
