|
Bone Ash
Bone ash is a white material produced by the calcination of bones. Typical bone ash consists of about 55.82% calcium oxide, 42.39% phosphorus pentoxide, and 1.79% water. The exact composition of these compounds varies depending upon the type of bones being used, but generally the formula for bone ash is: Ca5(OH)(PO4)3. Bone ash usually has a density around 3.10 g/mL and a melting point of 1670 °C (3038 °F). Most bones retain their cellular structure through calcination. History Bible From Isaiah: "And the people shall be as the burnings of lime: as thorns cut up shall they be burned in the fire" Its use is mentioned in the Book of Amos (2:1): "I will not turn away the punishment thereof, because he burned the bones of the King of Edom into lime." It was used in ancient formulas for white paint and cosmetic pigments, and in the cupellation process to separate silver from lead. Antiquity Burnt bones have been recovered from numerous Ancient Greek sanctuaries dating fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcination
Calcination refers to thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition. The root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime). This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smelting. Industrial calcination generally emits carbon dioxide (), making it a major contributor to climate change. A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
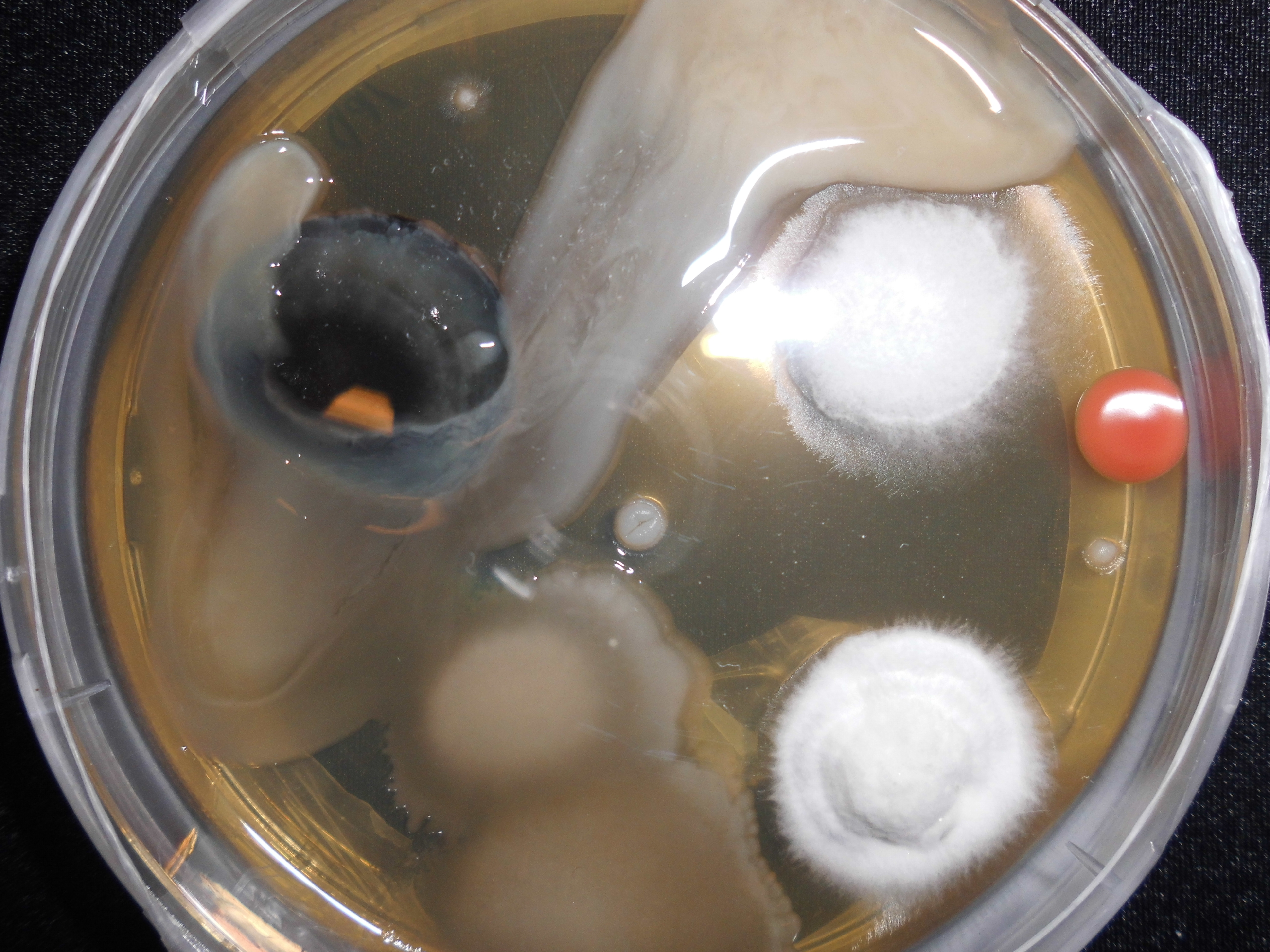
Cannibalism
Cannibalism is the act of consuming another individual of the same species as food. Cannibalism is a common ecological interaction in the animal kingdom and has been recorded in more than 1,500 species. Human cannibalism is well documented, both in ancient and in recent times. The rate of cannibalism increases in nutritionally poor environments as individuals turn to members of their own species as an additional food source.Elgar, M.A. & Crespi, B.J. (1992) ''Cannibalism: ecology and evolution among diverse taxa'', Oxford University Press, Oxford ngland New York. Cannibalism regulates population numbers, whereby resources such as food, shelter and territory become more readily available with the decrease of potential competition. Although it may benefit the individual, it has been shown that the presence of cannibalism decreases the expected survival rate of the whole population and increases the risk of consuming a relative. Other negative effects may include the increased r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be "miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', vo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superphosphate
Triple superphosphate is a component of fertilizer that primarily consists of monocalcium phosphate, Ca(H2PO4)2. Triple superphosphate is obtained by treating phosphate rock with phosphoric acid. Traditional routes for extraction of phosphate rock uses sulfuric acid gives single superphosphate, an approximate 1:1 mixture of Ca(H2PO4)2 and CaSO4 phosphogypsum). Double superphosphate refers to some average of triple- and single superphosphate, resulting from the extraction of phosphate rock with a mixture of phosphoric and sulfuric acids. Many fertilizers are derived from triple superphosphate, e.g. by blending with ammonium sulfate and potassium chloride Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. It is odorless and has a white or colorless vitreous crystal appearance. The solid dissolves readily in water, and its solutions have a salt .... Typical fertilizer-grade triple superphosphate contains 45% P2O5eq, single supe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfuric Acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formula . It is a colorless, odorless and viscous liquid that is miscible with water. Pure sulfuric acid does not exist naturally on Earth due to its strong affinity to water vapor; it is hygroscopic and readily absorbs water vapor from the air. Concentrated sulfuric acid is highly corrosive towards other materials, from rocks to metals, since it is an oxidant with powerful dehydrating properties. Phosphorus pentoxide is a notable exception in that it is not dehydrated by sulfuric acid, but to the contrary dehydrates sulfuric acid to sulfur trioxide. Upon addition of sulfuric acid to water, a considerable amount of heat is released; thus the reverse procedure of adding water to the acid should not be performed since the heat released may boi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Fertilizer
Organic fertilizers are fertilizers that are naturally produced. Fertilizers are materials that can be added to soil or plants, in order to provide nutrients and sustain growth. Typical organic fertilizers include all animal waste including meat processing waste, manure, slurry, and guano; plus plant based fertilizers such as compost; and biosolids. Inorganic "organic fertilizers" include minerals and ash. The organic-mess refers to the Principles of Organic Agriculture, which determines whether a fertilizer can be used for commercial organic agriculture, not whether the fertilizer consists of organic compounds. Examples and sources The main organic fertilizers are, peat, animal wastes, plant wastes from agriculture, and treated sewage sludge.Heinrich Dittmar, Manfred Drach, Ralf Vosskamp, Martin E. Trenkel, Reinhold Gutser, Günter Steffens "Fertilizers, 2. Types" in Ullmann's Encyclopedia of Industrial Chemistry, 2009, Wiley-VCH, Weinheim. Minerals Minerals can be mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicalcium Phosphate
Dicalcium phosphate is the calcium phosphate with the formula CaHPO4 and its dihydrate. The "di" prefix in the common name arises because the formation of the HPO42– anion involves the removal of two protons from phosphoric acid, H3PO4. It is also known as dibasic calcium phosphate or calcium monohydrogen phosphate. Dicalcium phosphate is used as a food additive, it is found in some toothpastes as a polishing agent and is a biomaterial. Preparation Dibasic calcium phosphate is produced by the neutralization of calcium hydroxide with phosphoric acid, which precipitates the dihydrate as a solid. At 60 °C the anhydrous form is precipitated: To prevent degradation that would form hydroxyapatite, sodium pyrophosphate or trimagnesium phosphate octahydrate are added when for example, dibasic calcium phosphate dihydrate is to be used as a polishing agent in toothpaste. In a continuous process CaCl2 can be treated with (NH4)2HPO4 to form the dihydrate: A slurry of the dihydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anorthite
Anorthite is the calcium endmember of the plagioclase feldspar mineral series. The chemical formula of pure anorthite is Ca Al2 Si2O8. Anorthite is found in mafic igneous rocks. Anorthite is rare on the Earth but abundant on the Moon. Mineralogy Anorthite is the calcium-rich endmember of the plagioclase solid solution series, the other endmember being albite (NaAlSi3O8). Anorthite also refers to plagioclase compositions with more than 90 molecular percent of the anorthite endmember. At standard pressure, anorthite melts at .J.R. Goldsmith (1980): The melting and breakdown reactions of anorthite at high pressures and temperatures. Am. Mineralogist. 65, 272-284, http://www.minsocam.org/ammin/AM65/AM65_272.pdf Occurrence Anorthite is a rare compositional variety of plagioclase. It occurs in mafic igneous rock. It also occurs in metamorphic rocks of granulite facies, in metamorphosed carbonate rocks, and corundum deposits. Its type localities are Monte Somma and Valle di Fass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tricalcium Phosphate
Tricalcium phosphate (sometimes abbreviated TCP) is a calcium salt of phosphoric acid with the chemical formula Ca3(PO4)2. It is also known as tribasic calcium phosphate and bone phosphate of lime (BPL). It is a white solid of low solubility. Most commercial samples of "tricalcium phosphate" are in fact hydroxyapatite. It exists as three crystalline polymorphs α, α′, and β. The α and α′ states are stable at high temperatures. Nomenclature ''Calcium phosphate'' refers to numerous materials consisting of calcium ions (Ca2+) together with orthophosphates (), metaphosphates or pyrophosphates () and occasionally oxide and hydroxide ions. Especially, the common mineral apatite has formula Ca5(PO4)3''X'', where ''X'' is F, Cl, OH, or a mixture; it is hydroxyapatite if the extra ion is mainly hydroxide. Much of the "tricalcium phosphate" on the market is actually powdered hydroxyapatite. Preparation Tricalcium phosphate is produced commercially by treating hydroxyapatite w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the triv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterilization (microbiology)
Sterilization refers to any process that removes, kills, or deactivates all forms of life (particularly microorganisms such as fungi, bacteria, spores, and unicellular eukaryotic organisms) and other biological agents such as prions present in or on a specific surface, object, or fluid. Sterilization can be achieved through various means, including heat, chemicals, irradiation, high pressure, and filtration. Sterilization is distinct from disinfection, sanitization, and pasteurization, in that those methods reduce rather than eliminate all forms of life and biological agents present. After sterilization, an object is referred to as being sterile or aseptic. Applications Foods One of the first steps toward modernized sterilization was made by Nicolas Appert, who discovered that application of heat over a suitable period slowed the decay of foods and various liquids, preserving them for safe consumption for a longer time than was typical. Canning of foods is an extension of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Matter
Organic matter, organic material, or natural organic matter refers to the large source of carbon-based compounds found within natural and engineered, terrestrial, and aquatic environments. It is matter composed of organic compounds that have come from the feces and remains of organisms such as plants and animals. Organic molecules can also be made by chemical reactions that do not involve life. Basic structures are created from cellulose, tannin, cutin, and lignin, along with other various proteins, lipids, and carbohydrates. Organic matter is very important in the movement of nutrients in the environment and plays a role in water retention on the surface of the planet. Formation Living organisms are composed of organic compounds. In life, they secrete or excrete organic material into their environment, shed body parts such as leaves and roots and after organisms die, their bodies are broken down by bacterial and fungal action. Larger molecules of organic matter can be formed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


2portion.jpg)