|
Bipyrimidine
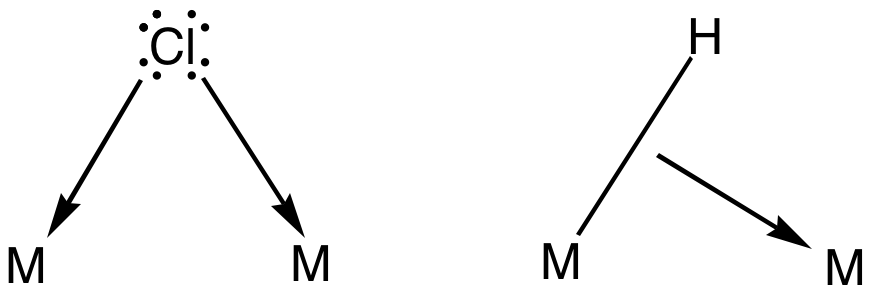
2,2′-Bipyrimidine is an organic compound with the formula (C4H3N2)2. It is a derivative of the heterocycle pyrimidine. It is a white solid. The compound is used as a bridging ligand in coordination chemistry. 2,2′-Bipyrimidines can be prepared by Ullmann coupling The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides. Traditionally this reaction is effected by copper, but palladium and nickel are also effective catalysts. The reaction is named after Fritz Ullmann. Mechanism ... of 2-iodopyrimidines. References {{DEFAULTSORT:Bipyrimidine, 2,2'- Chelating agents Pyrimidines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different chemical element, elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of these heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of heterocyclic chemistry focuses especially on unsaturated derivatives, and the preponderance of work and applications involves unstrained 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridine, thiophene, pyrrole, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine (nitrogen atoms at the 1 and 4 positions) and pyridazine (nitrogen atoms at the 1 and 2 positions). In nucleic acids, three types of nucleobases are pyrimidine derivatives: cytosine (C), thymine (T), and uracil (U). Occurrence and history The pyrimidine ring system has wide occurrence in nature as substituted and ring fused compounds and derivatives, including the nucleotides cytosine, thymine and uracil, thiamine (vitamin B1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine. Although pyrimidine derivatives such as alloxan were known in the early 19th century, a laboratory synthesis of a pyrimidine was not carried out until 1879, when Grimaux reported the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ullmann Coupling
The Ullmann reaction or Ullmann coupling is a coupling reaction between aryl halides. Traditionally this reaction is effected by copper, but palladium and nickel are also effective catalysts. The reaction is named after Fritz Ullmann. Mechanism The mechanism of the Ullmann reaction is extensively studied. Complications arise because the reactions are often heterogeneous. With copper as the halide acceptor, organocopper intermediates are invoked. Scope A typical example of classic Ullmann biaryl coupling is the conversion of ''ortho''-chloronitrobenzene into 2,2'-dinitrobiphenyl with a copper - bronze alloy. : : The traditional version of the Ullmann reaction requires harsh reaction conditions, and the reaction has a reputation for erratic yields. Because of these problems many improvements and alternative procedures have been introduced. The classical Ullmann reaction is limited to electron deficient aryl halides and requires harsh reaction conditions. Modern variants of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelating Agents
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |