|
Biocompatibility
Biocompatibility is related to the behavior of biomaterials in various contexts. The term refers to the ability of a material to perform with an appropriate host response in a specific situation. The ambiguity of the term reflects the ongoing development of insights into how biomaterials interact with the human body and eventually how those interactions determine the clinical success of a medical device (such as pacemaker, hip replacement or stent). Modern medical devices and prostheses are often made of more than one material so it might not always be sufficient to talk about the biocompatibility of a specific material. Since the immune response and repair functions in the body are so complicated it is not adequate to describe the biocompatibility of a single material in relation to a single cell type or tissue. Sometimes one hears of biocompatibility testing that is a large battery of in vitro test that is used in accordance with ISO 10993 (or other similar standards) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomaterials
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose – either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one. The corresponding field of study, called biomaterials science or biomaterials engineering, is about fifty years old. It has experienced steady growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. A biomaterial is different from a biological material, such as bone, that is produced by a biological system. However, "biomaterial" and "biological material" are often used interchangeably. Further, the word "bioterial" has been proposed as a potential alternate word for biologically-produced materials such as bone, or fungal biocomposites. Additionally, care should be exercised in defini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomaterial
A biomaterial is a substance that has been Biological engineering, engineered to interact with biological systems for a medical purpose – either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a Medical diagnosis, diagnostic one. The corresponding field of study, called biomaterials science or biomaterials engineering, is about fifty years old. It has experienced steady growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. A biomaterial is different from a biological material, such as bone, that is produced by a biological system. However, "biomaterial" and "biological material" are often used interchangeably. Further, the word "bioterial" has been proposed as a potential alternate word for biologically-produced materials such as bone, or fungal biocomposites. Additi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biocompatible Material
A biomaterial is a substance that has been engineered to interact with biological systems for a medical purpose – either a therapeutic (treat, augment, repair, or replace a tissue function of the body) or a diagnostic one. The corresponding field of study, called biomaterials science or biomaterials engineering, is about fifty years old. It has experienced steady growth over its history, with many companies investing large amounts of money into the development of new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering and materials science. A biomaterial is different from a biological material, such as bone, that is produced by a biological system. However, "biomaterial" and "biological material" are often used interchangeably. Further, the word "bioterial" has been proposed as a potential alternate word for biologically-produced materials such as bone, or fungal biocomposites. Additionally, care should be exercised in defini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Device
A medical device is any device intended to be used for medical purposes. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assurance before regulating governments allow marketing of the device in their country. As a general rule, as the associated risk of the device increases the amount of testing required to establish safety and efficacy also increases. Further, as associated risk increases the potential benefit to the patient must also increase. Discovery of what would be considered a medical device by modern standards dates as far back as in Baluchistan where Neolithic dentists used flint-tipped drills and bowstrings. Study of Archaeology, archeology and Roman medical literature also indicate that many types of medical devices were in widespread use during the time of ancient Rome. In the United States it was not until the Federal Food, Drug, and Cosmetic Act ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Implant (medicine)
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. For example, an implant may be a rod, used to strengthen weak bones. Medical implants are human-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In 2018, for example, American Elements developed a nickel alloy powder for 3D printing robust, long-lasting, and biocompatible medical implants. In some cases implants contain electronics, e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents. Applications Implants can roughly be categorized into groups by application: Sensory and neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Biocompatibility
Titanium was first introduced into surgeries in the 1950s after having been used in dentistry for a decade prior. It is now the metal of choice for prosthetics, internal fixation, inner body devices, and instrumentation. Titanium is used from head to toe in biomedical implants. One can find titanium in neurosurgery, bone conduction hearing aids, false eye implants, spinal fusion cages, pacemakers, toe implants, and shoulder/elbow/hip/knee replacements along with many more. The main reason why titanium is often used in the body is due to titanium's biocompatibility and, with surface modifications, bioactive surface. The surface characteristics that affect biocompatibility are surface texture, Steric effects#Steric hindrance, steric hindrance, binding sites, and hydrophobicity (wetting). These characteristics are optimized to create an ideal cellular response. Importantly, patient condition can influence the type of modification necessary, for instance in patients with steatotic liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bovine Submaxillary Mucin Coatings
300px, BSM can be derived from any bovine source. , right Bovine submaxillary mucin (BSM) coatings are a surface treatment provided to biomaterials intended to reduce the growth of disadvantageous bacteria and fungi such as '' S. epidermidis'', ''E. coli'', and ''Candida albicans''. BSM is a substance extracted from the fresh salivary glands of cows. It exhibits unique physical properties, such as high molecular weight and amphiphilicity, that allow it to be used for many biomedical applications. Each species possesses mucin-secreting submaxillary glands. Currently, eight different mucins have been identified for humans."Polymeric Biomaterials, Revised and Expanded." Google Books. Ed. Severian Dumitriu. N.p., n.d. Web. 5 May 2013. However, it is the mucin from bovine and porcine sources that have been used in several biomaterial applications. The most common use of BSM is in coatings for implanted materials. In such applications, the adsorption characteristics of BSM are integ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
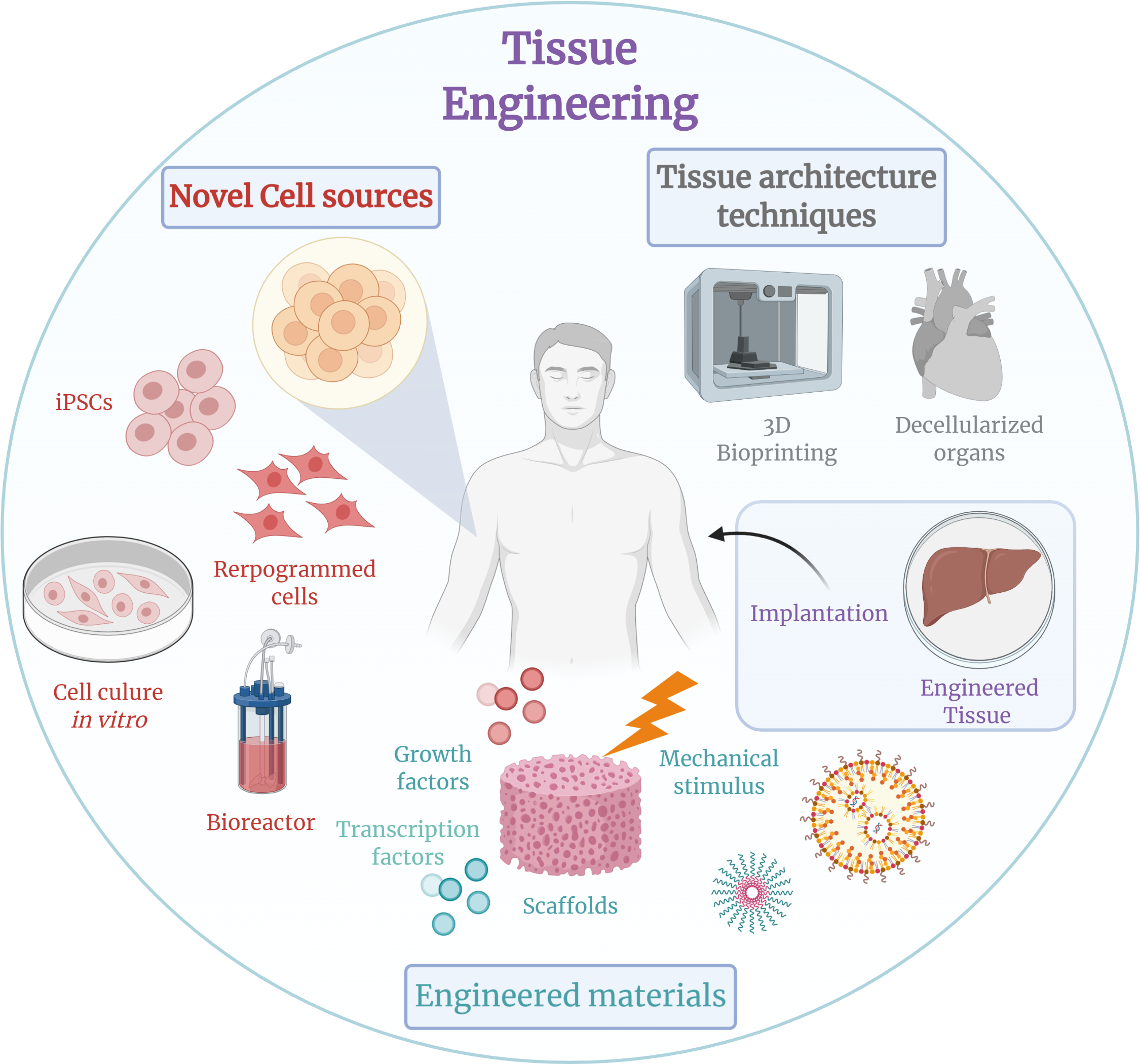
Tissue Engineering
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can be considered as a field of its own. While most definitions of tissue engineering cover a broad range of applications, in practice, the term is closely associated with applications that repair or replace portions of or whole tissues (i.e. organs, bone, cartilage, blood vessels, bladder, skin, muscle etc.). Often, the tissues involved require certain mechanical and structural properties for proper functioning. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Implant (medicine)
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. For example, an implant may be a rod, used to strengthen weak bones. Medical implants are human-made devices, in contrast to a transplant, which is a transplanted biomedical tissue. The surface of implants that contact the body might be made of a biomedical material such as titanium, silicone, or apatite depending on what is the most functional. In 2018, for example, American Elements developed a nickel alloy powder for 3D printing robust, long-lasting, and biocompatible medical implants. In some cases implants contain electronics, e.g. artificial pacemaker and cochlear implants. Some implants are bioactive, such as subcutaneous drug delivery devices in the form of implantable pills or drug-eluting stents. Applications Implants can roughly be categorized into groups by application: Sensory and neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vitro
''In vitro'' (meaning ''in glass'', or ''in the glass'') Research, studies are performed with Cell (biology), cells or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and its subdisciplines are traditionally done in labware such as test tubes, flasks, Petri dishes, and microtiter plates. Studies conducted using components of an organism that have been isolated from their usual biological surroundings permit a more detailed or more convenient analysis than can be done with whole organisms; however, results obtained from ''in vitro'' experiments may not fully or accurately predict the effects on a whole organism. In contrast to ''in vitro'' experiments, ''in vivo'' studies are those conducted in living organisms, including humans, known as clinical trials, and whole plants. Definition ''In vitro'' (Latin language, Latin for "in glass"; often not italicized in English usage) studies are conducted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |