|
Bamlanivimab Etesevimab
Bamlanivimab is a monoclonal antibody developed by AbCellera Biologics and Eli Lilly as a treatment for COVID-19. The medication was granted an emergency use authorization (EUA) by the US Food and Drug Administration (FDA) in November 2020, and the EUA was revoked in April 2021. Bamlanivimab is an IgG1 monoclonal antibody (mAb) directed against the spike protein of SARS-CoV-2. The aim is to block viral attachment and entry into human cells, thus neutralizing the virus, and help preventing and treating COVID-19. Bamlanivimab emerged from the collaboration between Lilly and AbCellera to create antibody therapies for the prevention and treatment of COVID-19. Bamlanivimab is also used as part of the bamlanivimab/etesevimab combination that was granted an EUA by the FDA. In June 2021, the US Office of the Assistant Secretary for Preparedness and Response (ASPR) paused distribution of bamlanivimab and etesevimab together, and etesevimab alone (to pair with existing supply of ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coronavirus Spike Protein
Spike (S) glycoprotein (sometimes also called spike protein, formerly known as E2) is the largest of the four major structural proteins found in coronaviruses. The spike protein assembles into trimers that form large structures, called spikes or peplomers, that project from the surface of the virion. The distinctive appearance of these spikes when visualized using negative stain transmission electron microscopy, "recalling the solar corona", gives the virus family its name. The function of the spike glycoprotein is to mediate viral entry into the host cell by first interacting with molecules on the exterior cell surface and then fusing the viral and cellular membranes. Spike glycoprotein is a class I fusion protein that contains two regions, known as S1 and S2, responsible for these two functions. The S1 region contains the receptor-binding domain that binds to receptors on the cell surface. Coronaviruses use a very diverse range of receptors; SARS-CoV (which causes SARS) an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bamlanivimab/etesevimab
Bamlanivimab/etesevimab is a combination of two monoclonal antibodies, bamlanivimab and etesevimab, administered together via intravenous infusion as a treatment for COVID-19. Both types of antibody target the surface spike protein of SARS‑CoV‑2. Bamlanivimab and etesevimab, administered together, are authorized in the United States for the treatment of mild-to-moderate COVID-19 in people aged twelve years of age and older weighing at least with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death. They are also authorized, when administered together, for use after exposure to the SARS-CoV-2 virus for post-exposure prophylaxis (PEP) for COVID-19 and are not authorized for pre-exposure prophylaxis to prevent COVID-19 before being exposed to the SARS-CoV-2 virus. In January 2022, the U.S. Food and Drug Administration (FDA) revised the authorizations for two monoclonal antibody ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiviral Drugs
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic (also termed antibacterial), antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees. Medical uses Most of the antiviral drugs now available are designed to help deal with HIV, herpes viruses, the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etesevimab
Bamlanivimab/etesevimab is a combination of two monoclonal antibodies, bamlanivimab and etesevimab, administered together via intravenous infusion as a treatment for COVID-19. Both types of antibody target the surface spike protein of SARS‑CoV‑2. Bamlanivimab and etesevimab, administered together, are authorized in the United States for the treatment of mild-to-moderate COVID-19 in people aged twelve years of age and older weighing at least with positive results of direct SARS-CoV-2 viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death. They are also authorized, when administered together, for use after exposure to the SARS-CoV-2 virus for post-exposure prophylaxis (PEP) for COVID-19 and are not authorized for pre-exposure prophylaxis to prevent COVID-19 before being exposed to the SARS-CoV-2 virus. In January 2022, the U.S. Food and Drug Administration (FDA) revised the authorizations for two monoclonal anti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emergency Use Authorization
An Emergency Use Authorization (EUA) in the United States is an authorization granted to the Food and Drug Administration (FDA) under sections of the Federal Food, Drug, and Cosmetic Act as added to and amended by various Act of Congress, Acts of Congress, including by the Pandemic and All-Hazards Preparedness Reauthorization Act of 2013 (PAHPRA), as codified by , to allow the use of a drug prior to approved drug, approval. It does not constitute ''approval'' of the drug in the full statutory meaning of the term, but instead authorizes the FDA to facilitate availability of an unapproved product, or an unapproved use of an approved product, during a declared state of emergency from one of several agencies or of a "material threat" by the Secretary of Homeland Security. Use EUAs have historically been infrequent. A review article by Rizk et al. provides a summary of the US experience in 2020 with pharmacological EUA approvals during the COVID-19 pandemic. It also provides a descripti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Operation Warp Speed
Operation Warp Speed (OWS) was a public–private partnership initiated by the United States government to facilitate and accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics. The first news report of Operation Warp Speed was on April 29, 2020, and the program was officially announced on May 15, 2020. It was headed by Moncef Slaoui from May 2020 to January 2021 and by David A. Kessler from January to February 2021. At the end of February 2021, Operation Warp Speed was transferred into the responsibilities of the White House COVID-19 Response Team. The program promoted mass production of multiple vaccines, and different types of vaccine technologies, based on preliminary evidence, allowing for faster distribution if clinical trials confirm one of the vaccines is safe and effective. The plan anticipated that some of these vaccines will not prove safe or effective, making the program more costly than typical vaccine develo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
End Organ Damage
End organ damage usually refers to damage occurring in major organs fed by the circulatory system (heart, kidneys, brain, eyes) which can sustain damage due to uncontrolled hypertension, hypotension, or hypovolemia. Evidence of hypertensive damage In the context of hypertension, features include: * Heart - evidence on electrocardiogram screening of the heart muscle thickening (but may also be seen on chest X-ray) suggesting left ventricular hypertrophy) or by echocardiography of less efficient function (left ventricular failure). * Brain- hypertensive encephalopathy, hemorrhagic stroke, subarachnoid hemorrhage, confusion, loss of consciousness, eclampsia, seizures, or transient ischemic attack. * Kidney - leakage of protein into the urine (albuminuria or proteinuria), or reduced renal function, hypertensive nephropathy, acute renal failure, or glomerulonephritis. * Eye - evidence upon fundoscopic examination of hypertensive retinopathy, retinal hemorrhage, papilledema and bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institutes Of Health
The National Institutes of Health, commonly referred to as NIH (with each letter pronounced individually), is the primary agency of the United States government responsible for biomedical and public health research. It was founded in the late 1880s and is now part of the United States Department of Health and Human Services. The majority of NIH facilities are located in Bethesda, Maryland, and other nearby suburbs of the Washington metropolitan area, with other primary facilities in the Research Triangle Park in North Carolina and smaller satellite facilities located around the United States. The NIH conducts its own scientific research through the NIH Intramural Research Program (IRP) and provides major biomedical research funding to non-NIH research facilities through its Extramural Research Program. , the IRP had 1,200 principal investigators and more than 4,000 postdoctoral fellows in basic, translational, and clinical research, being the largest biomedical research instit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
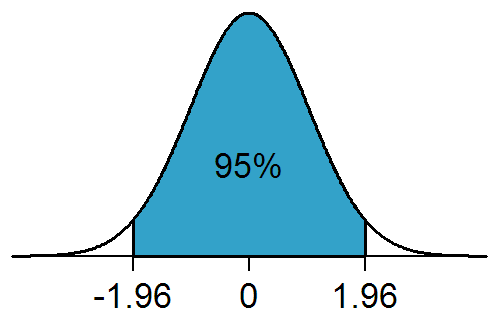
Statistical Significance
In statistical hypothesis testing, a result has statistical significance when it is very unlikely to have occurred given the null hypothesis (simply by chance alone). More precisely, a study's defined significance level, denoted by \alpha, is the probability of the study rejecting the null hypothesis, given that the null hypothesis is true; and the ''p''-value of a result, ''p'', is the probability of obtaining a result at least as extreme, given that the null hypothesis is true. The result is statistically significant, by the standards of the study, when p \le \alpha. The significance level for a study is chosen before data collection, and is typically set to 5% or much lower—depending on the field of study. In any experiment or observation that involves drawing a sample from a population, there is always the possibility that an observed effect would have occurred due to sampling error alone. But if the ''p''-value of an observed effect is less than (or equal to) the significanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)
_(cropped).jpg)