|
Astacin
Astacins are a family of multidomain metalloendopeptidases which are either secreted or membrane-anchored. These metallopeptidases belong to the MEROPS peptidase family M12, subfamily M12A (astacin family, clan MA(M)). The protein fold of the peptidase domain for members of this family resembles that of thermolysin, the type example for clan MA and the predicted active site residues for members of this family and thermolysin occur in the motif HEXXH. The astacin family of metalloendopeptidases (EC 3.4.24.21) encompasses a range of proteins found in hydra to humans, in mature and developmental systems. Their functions include activation of growth factors, degradation of polypeptides, and processing of extracellular proteins. The proteins are synthesised with N-terminal signal and pro-enzyme sequences, and many contain multiple domains C-terminal to the protease domain. They are either secreted from cells, or are associated with the plasma membrane. The astacin molecule adopts a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MMPs
Matrix metalloproteinases (MMPs), also known as matrix metallopeptidases or matrixins, are metalloproteinases that are calcium-dependent zinc-containing endopeptidases; other family members are adamalysins, serralysins, and astacins. The MMPs belong to a larger family of proteases known as the metzincin superfamily. Collectively, these enzymes are capable of degrading all kinds of extracellular matrix proteins, but also can process a number of bioactive molecules. They are known to be involved in the cleavage of cell surface receptors, the release of apoptotic ligands (such as the FAS ligand), and chemokine/cytokine inactivation. MMPs are also thought to play a major role in cell behaviors such as cell proliferation, migration (adhesion/dispersion), differentiation, angiogenesis, apoptosis, and host defense. They were first described in vertebrates (1962), including humans, but have since been found in invertebrates and plants. They are distinguished from other endopeptidas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloendopeptidase
A metalloendopeptidase is an enzyme that functions as a metalloproteinase endopeptidase Endopeptidase or endoproteinase are proteolytic peptidases that break peptide bonds of nonterminal amino acids (i.e. within the molecule), in contrast to exopeptidases, which break peptide bonds from end-pieces of terminal amino acids. For this re .... References External links * EC 3.4.24 {{enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidazole ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism of carbohydrates, fats and protein by promoting the absorption of glucose from the blood into liver, fat and skeletal muscle cells. In these tissues the absorbed glucose is converted into either glycogen via glycogenesis or fats (triglycerides) via lipogenesis, or, in the case of the liver, into both. Glucose production and secretion by the liver is strongly inhibited by high concentrations of insulin in the blood. Circulating insulin also affects the synthesis of proteins in a wide variety of tissues. It is therefore an anabolic hormone, promoting the conversion of small molecules in the blood into large molecules inside the cells. Low insulin levels in the blood have the opposite effect by promoting widespread catabolism, especially o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
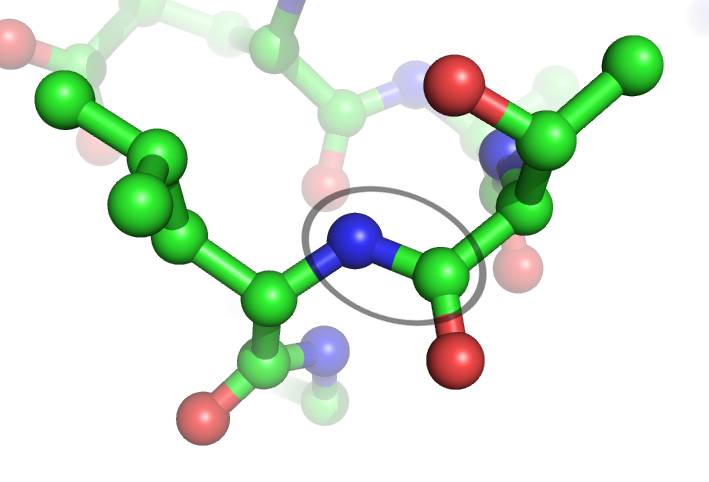
Peptide Bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein chain. It can also be called a eupeptide bond to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Synthesis When two amino acids form a ''dipeptide'' through a ''peptide bond'', it is a type of condensation reaction. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−). The two joined amino acids are called a dipeptide. The am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Venom (poison)
Venom or zootoxin is a type of toxin produced by an animal that is actively delivered through a wound by means of a bite, sting, or similar action. The toxin is delivered through a specially evolved ''venom apparatus'', such as fangs or a stinger, in a process called envenomation. Venom is often distinguished from poison, which is a toxin that is passively delivered by being ingested, inhaled, or absorbed through the skin, and toxungen, which is actively transferred to the external surface of another animal via a physical delivery mechanism. Venom has evolved in terrestrial and marine environments and in a wide variety of animals: both predators and prey, and both vertebrates and invertebrates. Venoms kill through the action of at least four major classes of toxin, namely necrotoxins and cytotoxins, which kill cells; neurotoxins, which affect nervous systems; myotoxins, which damage muscles; and haemotoxins, which disrupt blood clotting. Venomous animals cause tens of thousa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Snake
Snakes are elongated, Limbless vertebrate, limbless, carnivore, carnivorous reptiles of the suborder Serpentes . Like all other Squamata, squamates, snakes are ectothermic, amniote vertebrates covered in overlapping Scale (zoology), scales. Many species of snakes have skulls with several more joints than their lizard ancestors, enabling them to swallow prey much larger than their heads (cranial kinesis). To accommodate their narrow bodies, snakes' paired organs (such as kidneys) appear one in front of the other instead of side by side, and most have only one functional lung. Some species retain a pelvic girdle with a pair of vestigial claws on either side of the cloaca. Lizards have evolved elongate bodies without limbs or with greatly reduced limbs about twenty-five times independently via convergent evolution, leading to many lineages of legless lizards. These resemble snakes, but several common groups of legless lizards have eyelids and external ears, which snakes lack, altho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serralysin
Serralysin (, ''Pseudomonas aeruginosa alkaline proteinase'', ''Escherichia freundii proteinase'', ''Serratia marcescens extracellular proteinase'', ''Serratia marcescens metalloproteinase'', ''Pseudomonas aeruginosa alk. protease'', ''Serratia marcescens metalloprotease'') is an enzyme. This enzyme catalyses the following chemical reaction : Preferential cleavage of bonds with hydrophobic residues in P1' This extracellular endopeptidase is present in ''Pseudomonas aeruginosa'', '' Escherichia freundii'', ''Serratia marcescens'' and ''Erwinia chrysanthemi ''Dickeya dadantii'' is a gram-negative bacillus that belongs to the family Pectobacteriaceae. It was formerly known as ''Erwinia chrysanthemi'' but was reassigned as ''Dickeya dadantii'' in 2005. Members of this family are facultative anaerobes ...''. References External links * {{Portal bar, Biology, border=no EC 3.4.24 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domains
In molecular biology, a protein domain is a region of a protein's polypeptide chain that is self-stabilizing and that folds independently from the rest. Each domain forms a compact folded three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or disulfide bridges. Domains often form functional units, such as the calcium-binding EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimeric proteins. Background The concept of the domain was first proposed in 1973 by Wetlaufer after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archetypal
The concept of an archetype (; ) appears in areas relating to behavior, historical psychology, and literary analysis. An archetype can be any of the following: # a statement, pattern of behavior, prototype, "first" form, or a main model that other statements, patterns of behavior, and objects copy, emulate, or "merge" into. Informal synonyms frequently used for this definition include "standard example", "basic example", and the longer-form "archetypal example"; mathematical archetypes often appear as "canonical examples". # the Platonic concept of ''pure form'', believed to embody the fundamental characteristics of a thing. # a collectively-inherited unconscious idea, a pattern of thought, image, etc., that is universally present, in individual psyches, as in Jungian psychology # a constantly-recurring symbol or motif in literature, painting, or mythology. This definition refers to the recurrence of characters or ideas sharing similar traits throughout various, seemingly un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-sheet
The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformation. The supramolecular association of β-sheets has been implicated in the formation of the fibrils and protein aggregates observed in amyloidosis, notably Alzheimer's disease. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids in order to build accurate models, especially since he did not then know that the peptide bond was planar. A refined versi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |