|
Antiperthite
Perthite is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na-rich feldspar domains separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perthite 0
Perthite is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na-rich feldspar domains separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
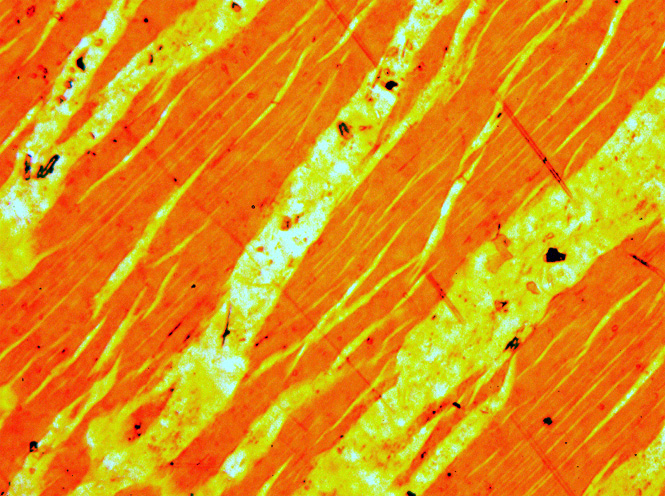
Perthitic Feldspar Dan Patch SD
Perthite is used to describe an intergrowth of two feldspars: a host grain of potassium-rich alkali feldspar (near K-feldspar, KAlSi3O8, in composition) includes exsolved lamellae or irregular intergrowths of sodic alkali feldspar (near albite, NaAlSi3O8, in composition). Typically, the host grain is orthoclase or microcline, and the lamellae are albite. If sodic feldspar is the dominant phase, the result is an antiperthite and where the feldspars are in roughly equal proportions the result is a mesoperthite. The intergrowth forms by exsolution due to cooling of a grain of alkali feldspar with a composition intermediate between K-feldspar and albite. There is complete solid solution between albite and K-feldspar at temperatures near 700 °C and pressures like those within the crust of the Earth, but a miscibility gap is present at lower temperatures. If an alkali feldspar grain with an intermediate composition cools slowly enough, K-rich and more Na-rich feldspar domains separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Solution
A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions - ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moonstone (gemstone)
Moonstone is a sodium potassium aluminium silicate ((Na,K)AlSi3O8) of the feldspar group that displays a pearly and opalescent schiller. An alternative name is hecatolite. Etymology File:Pierrelune.jpg, Moonstone polished ''en cabochon'' The name ''moonstone'' derives from the stone's characteristic visual effect, called adularescence (or schiller), which produces a milky, bluish interior light. This effect is caused by light diffraction through alternating layers of orthoclast and albite within the stone. The diffracted light varies from white to blue, depending on the thinness of the albite layers. More technically, this micro-structure consists of regular exsolution layers (lamellae) of different alkali feldspars (orthoclase and sodium-rich plagioclase). Polished moonstones often display chatoyancy ("cat's eye" effect), where a luminous streak appears through the stone. Asterism is rare and produces four-legged stars. Geology The most common moonstone is of the orthocl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amazonite
Amazonite, also known as Amazonstone, is a green tectosilicate mineral, a variety of the potassium feldspar called microcline. Its chemical formula is KAlSi3O8, which is polymorphic to orthoclase. Its name is taken from that of the Amazon River, from which green stones were formerly obtained, though it is unknown whether those stones were amazonite. Although it has been used for over two thousand years, as attested by archaeological finds in Egypt and Mesopotamia, no ancient or medieval authority mentions it. It was first described as a distinct mineral only in the 18th century.Mikhail Ostrooumov, ''Amazonite: Mineralogy, Crystal Chemistry, and Typomorphism'' (Elsevier, 2016), p. 1-12. Green and greenish-blue varieties of potassium feldspars that are predominantly triclinic are designated as amazonite. It has been described as a "beautiful crystallized variety of a bright verdigris-green" and as possessing a "lively green colour." It is occasionally cut and used as a gemstone. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
South Dakota
South Dakota (; Sioux language, Sioux: , ) is a U.S. state in the West North Central states, North Central region of the United States. It is also part of the Great Plains. South Dakota is named after the Lakota people, Lakota and Dakota people, Dakota Sioux Native Americans in the United States, Native American tribes, who comprise a large portion of the population with nine Indian reservation, reservations currently in the state and have historically dominated the territory. South Dakota is the List of U.S. states and territories by area, seventeenth largest by area, but the List of U.S. states and territories by population, 5th least populous, and the List of U.S. states and territories by population density, 5th least densely populated of the List of U.S. states, 50 United States. As the southern part of the former Dakota Territory, South Dakota became a state on November 2, 1889, simultaneously with North Dakota. They are the 39th and 40th states admitted to the union; Pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Miscibility Gap
A miscibility gap is a region in a phase diagram for a mixture of components where the mixture exists as two or more phases – any region of composition of mixtures where the constituents are not completely miscible. The IUPAC Gold Book defines ''miscibility gap'' as "Area within the coexistence curve of an isobaric phase diagram (temperature vs composition) or an isothermal phase diagram (pressure vs composition)." A miscibility gap between isostructural phases may be described as the ''solvus'', a term also used to describe the boundary on a phase diagram between a miscibility gap and other phases. Thermodynamically, miscibility gaps indicate a maximum (''e.g.'' of Gibbs energy) in the composition range. Named miscibility gaps A number of miscibility gaps in phase systems are named, including * The ''huttenlocher'' (found in bytownite, anorthite composition An55-95.), ''boggild'' (in labradorite, An39-48 and An53-63.) and peristerite (in oligoclase, ~An5-15.) miscibility g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exsolution
A solid solution, a term popularly used for metals, is a homogenous mixture of two different kinds of atoms in solid state and have a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solution" is used to describe the intimate mixing of components at the atomic level and distinguishes these homogeneous materials from physical mixtures of components. Two terms are mainly associated with solid solutions - ''solvents'' and ''solutes,'' depending on the relative abundance of the atomic species. In general if two compounds are isostructural then a solid solution will exist between the end members (also known as parents). For example sodium chloride and potassium chloride have the same cubic crystal structure so it is possible to make a pure compound with any ratio of sodium to potassium (Na1-xKx)Cl by dissolving that ratio of NaCl and KCl in water and then evaporating the solution. A member of this family is sold under t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feldspar
Feldspars are a group of rock-forming aluminium tectosilicate minerals, also containing other cations such as sodium, calcium, potassium, or barium. The most common members of the feldspar group are the ''plagioclase'' (sodium-calcium) feldspars and the ''alkali'' (potassium-sodium) feldspars. Feldspars make up about 60% of the Earth's crust, and 41% of the Earth's continental crust by weight. Feldspars crystalize from magma as both intrusive and extrusive igneous rocks and are also present in many types of metamorphic rock. Rock formed almost entirely of calcic plagioclase feldspar is known as anorthosite. Feldspars are also found in many types of sedimentary rocks. Compositions The feldspar group of minerals consists of tectosilicates, silicate minerals in which silicon ions are linked by shared oxygen ions to form a three-dimensional network. Compositions of major elements in common feldspars can be expressed in terms of three endmembers: * potassium feldspar (K-spar) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcline
Microcline (KAlSi3O8) is an important igneous rock-forming tectosilicate mineral. It is a potassium-rich alkali feldspar. Microcline typically contains minor amounts of sodium. It is common in granite and pegmatites. Microcline forms during slow cooling of orthoclase; it is more stable at lower temperatures than orthoclase. Sanidine is a polymorph of alkali feldspar stable at yet higher temperature. Microcline may be clear, white, pale-yellow, brick-red, or green; it is generally characterized by cross-hatch twinning that forms as a result of the transformation of monoclinic orthoclase into triclinic microcline. The chemical compound name is potassium aluminium silicate, and it is known as E number reference E555. Geology Microcline may be chemically the same as monoclinic orthoclase, but because it belongs to the triclinic crystal system, the prism angle is slightly less than right angles; hence the name "microcline" from the Greek "small slope." It is a fully ordered triclin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoclase
Orthoclase, or orthoclase feldspar (endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture," because its two cleavage planes are at right angles to each other. It is a type of potassium feldspar, also known as K-feldspar. The gem known as moonstone (see below) is largely composed of orthoclase. Formation and subtypes Orthoclase is a common constituent of most granites and other felsic igneous rocks and often forms huge crystals and masses in pegmatite. Typically, the pure potassium endmember of orthoclase forms a solid solution with albite, the sodium endmember (NaAlSi3O8), of plagioclase. While slowly cooling within the earth, sodium-rich albite lamellae form by exsolution, enriching the remaining orthoclase with potassium. The resulting intergrowth of the two feldspars is called perthite. The higher-temperature polymorph of KAlSi3O8 is sanidine. Sanidine is common in rapidly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |