|
Anthranilate Synthase
The enzyme anthranilate synthase () catalyzes the chemical reaction :chorismate + L-glutamine \rightleftharpoons anthranilate + pyruvate + L-glutamate Function Anthranilate synthase creates anthranilate, an important intermediate in the biosynthesis of indole, and by extension, the amino acid tryptophan. Tryptophan can then be metabolized further into serotonin, melatonin, or various auxins. Reaction Anthranilate synthase catalyzes the change from chorismate to anthranilate. As its other substrate, it can use either glutamine or ammonia. During the reaction, both a hydroxyl group and an enolpyruvyl group are removed from the aromatic ring. The enolpyruvyl group gains a proton to form pyruvate. It has been shown that the proton comes from the surrounding water and not from an intramolecular shift of a hydrogen atom on the substrates. The amino group of glutamine (or ammonia itself) attacks chorismate in position 2, which leads to the elimination of enolpyruvyl group from posit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
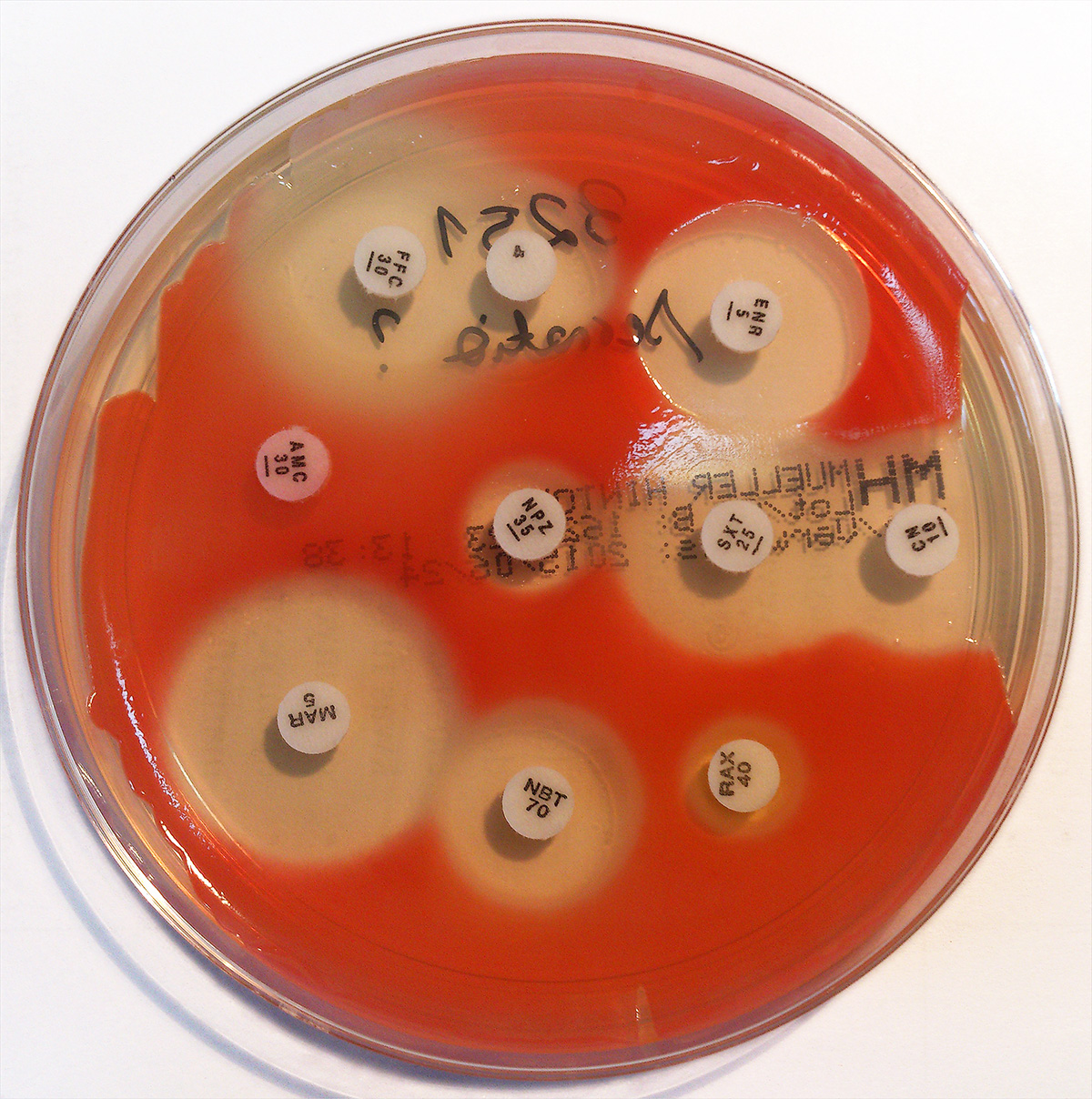
Serratia Marcescens
''Serratia marcescens'' () is a species of rod-shaped, Gram-negative bacteria in the family Yersiniaceae. It is a facultative anaerobe and an opportunistic pathogen in humans. It was discovered in 1819 by Bartolomeo Bizio in Padua, Italy.Serratia marcescens. (2011, April). Retrieved from https://microbewiki.kenyon.edu/index.php/Serratia_marcescens ''S. marcescens'' is commonly involved in hospital-acquired infections (HAIs), also called nosocomial infections, particularly catheter-associated bacteremia, urinary tract infections, and wound infections, and is responsible for 1.4% of HAI cases in the United States. It is commonly found in the respiratory and urinary tracts of hospitalized adults and in the gastrointestinal systems of children. Due to its abundant presence in the environment, and its preference for damp conditions, ''S. marcescens'' is commonly found growing in bathrooms (especially on tile grout, shower corners, toilet water lines, and basins), where it manife ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Paint
Reaction may refer to a process or to a response to an action, event, or exposure: Physics and chemistry * Chemical reaction * Nuclear reaction * Reaction (physics), as defined by Newton's third law * Chain reaction (other). Biology and medicine * Adverse drug reaction * Allergic reaction *Reflex, neural reaction * Hypersensitivity, immune reaction *Intolerance (other) *Light reaction (other). Psychology * Emotional, reaction * Reactivity (behaviour) *Proactivity, opposite of reactive behaviour * Reactive attachment disorder. Politics and culture *Reactionary In political science, a reactionary or a reactionist is a person who holds political views that favor a return to the ''status quo ante'', the previous political state of society, which that person believes possessed positive characteristics abse ..., a political tendency * Reaction video * Commentary (other). Proper names and titles * ''Reaction'' (album), a 1986 album by American ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules, such as proteins and nucleic acids. The data, typically obtained by X-ray crystallography, NMR spectroscopy, or, increasingly, cryo-electron microscopy, and submitted by biologists and biochemists from around the world, are freely accessible on the Internet via the websites of its member organisations (PDBe, PDBj, RCSB, and BMRB). The PDB is overseen by an organization called the Worldwide Protein Data Bank, wwPDB. The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other databases use protein structures deposited in the PDB. For example, SCOP and CATH classify protein structures, while PDBsum provides a graphic overview of PDB entries using information from other sources, such as Gene ontology. History Two force ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tertiary Structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may interact and bond in a number of ways. The interactions and bonds of side chains within a particular protein determine its tertiary structure. The protein tertiary structure is defined by its atomic coordinates. These coordinates may refer either to a protein domain or to the entire tertiary structure.Branden C. and Tooze J. "Introduction to Protein Structure" Garland Publishing, New York. 1990 and 1991. A number of tertiary structures may fold into a quaternary structure.Kyte, J. "Structure in Protein Chemistry." Garland Publishing, New York. 1995. History The science of the tertiary structure of proteins has progressed from one of hypothesis to one of detailed definition. Although Emil Fischer had suggested proteins were made of polypept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine, Tyrosine And Tryptophan Biosynthesis
Amino acid synthesis is the set of biochemical processes (metabolic pathways) by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids). α-Ketoglutarates: glutamate, glutamine, proline, arginine Most amino acids are synthesized from α- ketoacids, and later transaminated from another amino acid, usually glutamate. The enzyme involved in this reaction is an aminotransferase. : α-ketoacid + glutamate ⇄ amino acid + α-ketoglutarate Glutamate itself is formed by amination of α-ketoglutarate: : α-ketoglutarate + ⇄ glutamate The α-ketoglutarate family of amino acid synthesis (synthesis of glutamate, glutamine, proline and arginine) begins with α-ketoglutarate, an intermediate in the Citric Acid Cycle. The concentra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Enzymes
This article lists enzymes by their classification in the International Union of Biochemistry and Molecular Biology's Enzyme Commission (EC) numbering system. * List of EC numbers (EC 5) * List of EC numbers (EC 6) :Oxidoreductases (EC 1) (Oxidoreductase) *Dehydrogenase * Luciferase *DMSO reductase :EC 1.1 (act on the CH-OH group of donors) * :EC 1.1.1 (with NAD+ or NADP+ as acceptor) ** Alcohol dehydrogenase (NAD) ** Alcohol dehydrogenase (NADP) **Homoserine dehydrogenase ** Aminopropanol oxidoreductase **Diacetyl reductase **Glycerol dehydrogenase **Propanediol-phosphate dehydrogenase ** glycerol-3-phosphate dehydrogenase (NAD+) ** D-xylulose reductase **L-xylulose reductase **Lactate dehydrogenase **Malate dehydrogenase **Isocitrate dehydrogenase ** HMG-CoA reductase * :EC 1.1.2 (with a cytochrome as acceptor) * :EC 1.1.3 (with oxygen as acceptor) **Glucose oxidase **L-gulonolactone oxidase **Thiamine oxidase **Xanthine oxidase * :EC 1.1.4 (with a disul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking (an elimination reaction) of various chemical bonds by means other than hydrolysis (a substitution reaction) and oxidation, often forming a new double bond or a new ring structure. The reverse reaction is also possible (called a Michael reaction). For example, an enzyme that catalyzed this reaction would be a lyase: : ATP → cAMP + PPi Lyases differ from other enzymes in that they require only one substrate for the reaction in one direction, but two substrates for the reverse reaction. Nomenclature Systematic names are formed as "''substrate group-lyase''." Common names include decarboxylase, dehydratase, aldolase, etc. When the product is more important, synthase may be used in the name, e.g. phosphosulfolactate synthase (EC 4.4.1.19, Michael addition of sulfite to phosphoenolpyruvate). A combination of both an elimination and a Michael addition is seen in O-succinylhomoserine (thiol)-lyase (MetY or MetZ) which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
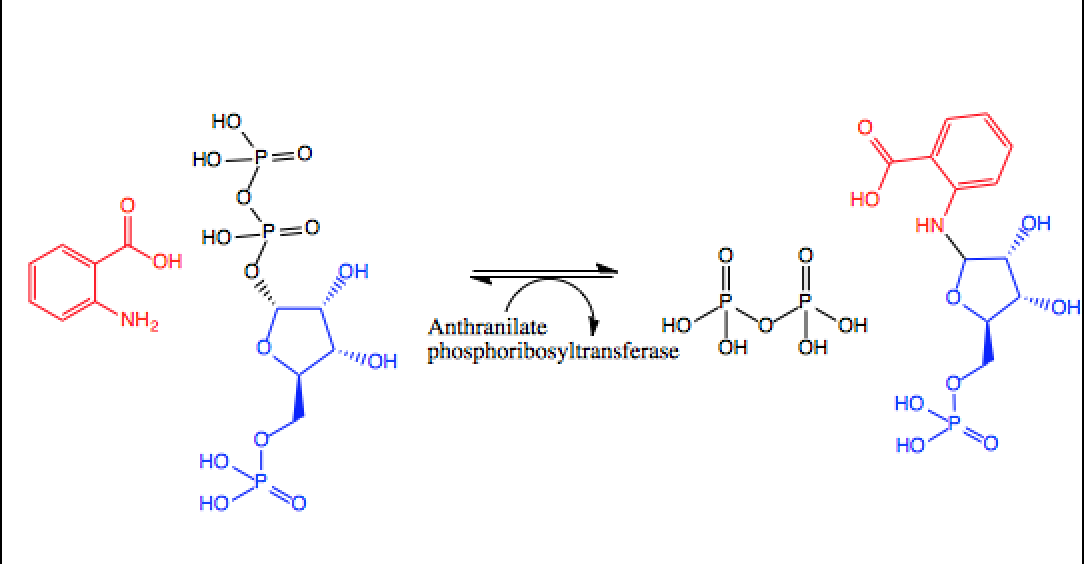
Anthranilate Phosphoribosyltransferase
In enzymology, an anthranilate phosphoribosyltransferase () is an enzyme that catalyzes the chemical reaction :anthranilate + phosphoribosyl pyrophosphate \rightleftharpoons N-(5-phosphoribosyl)-anthranilate + diphosphate The two substrates of this enzyme are anthranilate and phosphoribosyl pyrophosphate. Its two products are N-(5-phosphoribosyl)-anthranilate and diphosphate. This enzyme participates in aromatic amino acid biosynthesis and two-component system (general). Nomenclature This enzyme belongs to the family of glycosyltransferases, specifically the pentosyltransferases. The systematic name of this enzyme class is N-(5-phospho-D-ribosyl)-anthranilate:diphosphate phospho-alpha-D-ribosyltransferase. Other names in common use are: * anthranilate 5-phosphoribosylpyrophosphate * anthranilate phosphoribosylpyrophosphate phosphoribosyltransferase * anthranilate-PP-ribose-P phosphoribosyltransferase * phosphoribosyl-anthranilate pyrophosphorylase * phosphoribosylanthrani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enterobacteriaceae
Enterobacteriaceae is a large family (biology), family of Gram-negative bacteria. It was first proposed by Rahn in 1936, and now includes over 30 genera and more than 100 species. Its classification above the level of family is still a subject of debate, but one classification places it in the order Enterobacterales of the class Gammaproteobacteria in the phylum Pseudomonadota. In 2016, the description and members of this family were emended based on comparative genomic analyses by Adeolu et al. Enterobacteriaceae includes, along with many harmless Symbiosis, symbionts, many of the more familiar pathogenic bacteria, pathogens, such as ''Salmonella'', ''Escherichia coli'', ''Klebsiella'', and ''Shigella''. Other disease-causing bacteria in this family include ''Enterobacter'' and ''Citrobacter''. Members of the Enterobacteriaceae can be Bacterial taxonomy#Nomenclature, trivially referred to as enterobacteria or "enteric bacteria",as several members live in the intestines of anim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
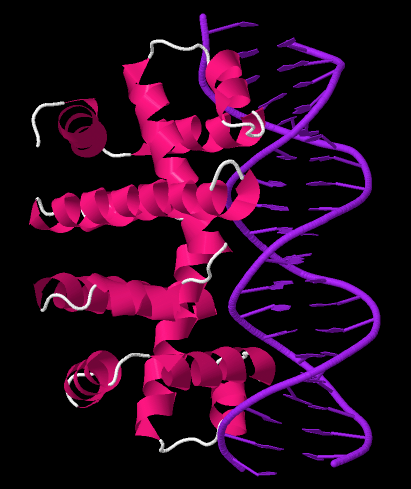
Trp Operon
The ''trp'' operon is a group of genes that are transcribed together, encoding the enzymes that produce the amino acid tryptophan in bacteria. The ''trp'' operon was first characterized in ''Escherichia coli,'' and it has since been discovered in many other bacteria. The operon is regulated so that, when tryptophan is present in the environment, the genes for tryptophan synthesis are repressed. The ''trp'' operon contains five structural genes: ''trpE'', ''trpD'', ''trpC'', ''trpB'', and ''trpA'', which encode the enzymes needed to synthesize tryptophan. It also contains a repressive regulator gene called trpR. When tryptophan is present, the trpR protein binds to the operator, blocking transcription of the ''trp'' operon by RNA polymerase. This operon is an example of repressible negative regulation of gene expression. The repressor protein binds to the operator in the presence of tryptophan (repressing transcription) and is released from the operon when tryptophan is absent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |