|
Ampy Dance Remix
2-Picolylamine is an organic compound with the formula H2NCH2C5H4N. A colorless liquid, it is a common bidentate ligand and a precursor to more complex multidentate ligands such as tris(2-pyridylmethyl)amine. It is usually prepared by hydrogenation of 2-cyanopyridine. One such complex is Baratta's catalyst RuCl2(PPh3)2(ampy) (ampy = 2-picolylamine) for transfer hydrogenation. Salts of the complex e(pyCH2NH2)3sup>2+ exhibit spin crossover behavior, whereby the complex switches from high to low spin configurations, depending on the temperature. Safety The oral in quail Quail is a collective name for several genera of mid-sized birds generally placed in the order Galliformes. The collective noun for a group of quail is a flock, covey, or bevy. Old World quail are placed in the family Phasianidae, and New Wor ... is low, being 750 mg/kg. References {{DEFAULTSORT:Picolylamine, 2- 2-Pyridyl compounds Amines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bidentate Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(2-pyridylmethyl)amine
Tris(2-pyridylmethyl)amine (abbreviated TPMA or TPA) is an organic compound with the formula (C5H4NCH2)3N. It is a tertiary amine with three picolyl substituents. It is a white solid that is soluble in polar organic solvents. It is a ligand in coordination chemistry. The ligand is prepared by the alkylation of 2-picolylamine by picolyl chloride:{{cite book , author1=James W. Canary , author2=Yihan Wang , author3=Richard Roy, Jr. , title = Tris 2-Pyridyl)MethylAmine (TPA) and (+)-Bis 2-Pyridyl)methyl1-(2-Pyridyl)-Ethylamine (α-Metpa) , journal = Inorg. Synth. , series=Inorganic Syntheses , year = 1998 , volume = 32 , pages = 70–75 , doi = 10.1002/9780470132630.ch11, isbn=9780470132630 :2 C5H4NCH2Cl + C5H4NCH2NH2 → (C5H4NCH2)3N + 2 HCl TPMA is a tripodal ligand, often used to simulate the coordination environment within some proteins. It is also used as a copper ligand in ATRP. Related ligands * dipicolylamine, an intermediate in the synthesis of TPMA. *2-pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the liquid phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
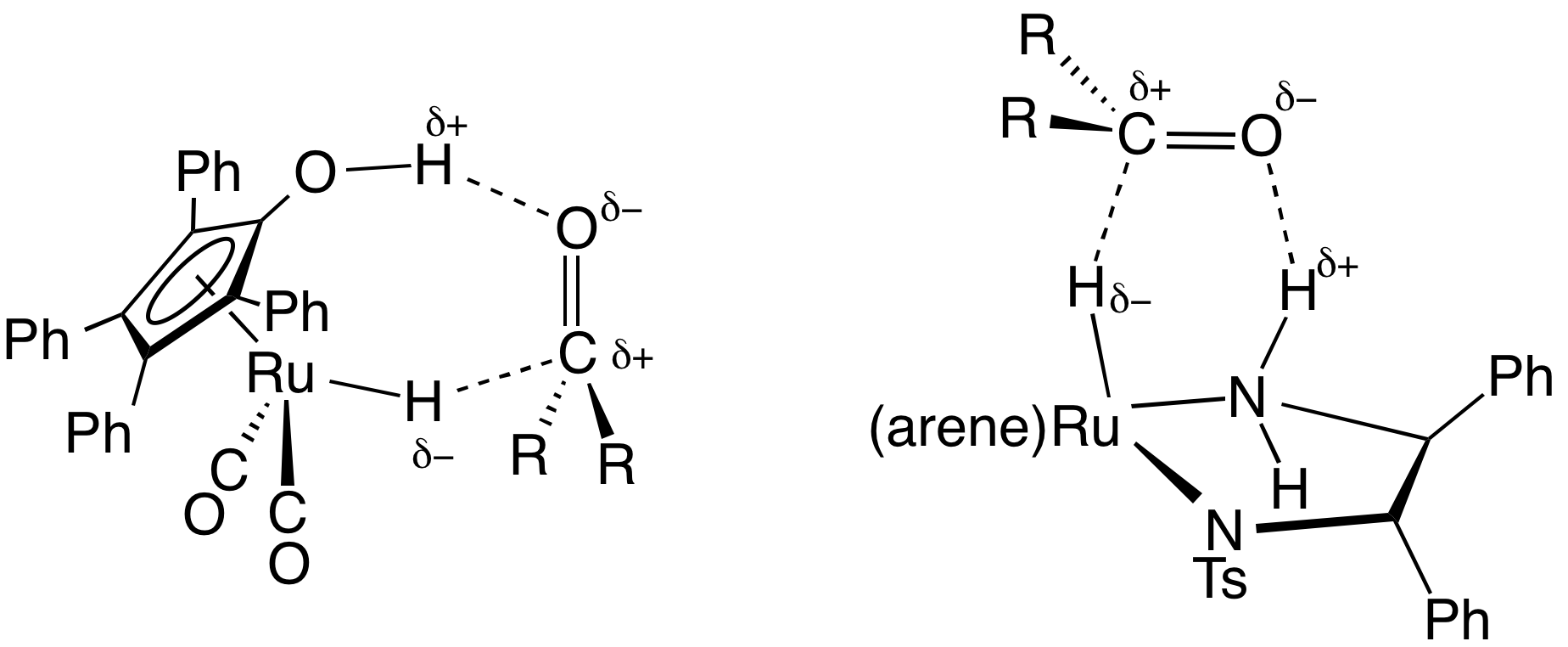
Transfer Hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to , acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin. Organometallic catalysts In the area of organic synthesis, a useful family of hydrogen-trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
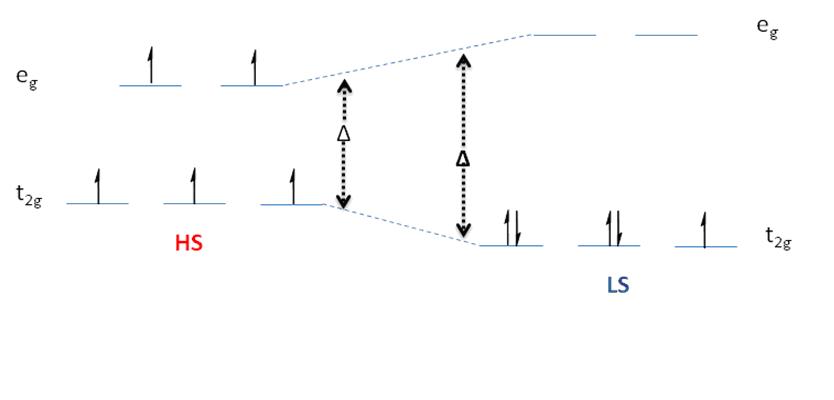
Spin Crossover
Spin crossover (SCO) is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to an external stimulus. The stimuli can include temperature or pressure. Spin crossover is sometimes referred to as spin transition or spin equilibrium behavior. The change in spin state usually involves interchange of low spin (LS) and high spin (HS) configuration. Spin crossover is commonly observed with first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves typically plot the high-spin molar fraction against temperature. Often a gradual spin transition is followed by an abrupt (ΔT = 10K) transition with hysteresis and a two-step transition. The abruptness with hysteresis indicates cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quail
Quail is a collective name for several genera of mid-sized birds generally placed in the order Galliformes. The collective noun for a group of quail is a flock, covey, or bevy. Old World quail are placed in the family Phasianidae, and New World quail are placed in the family Odontophoridae. The species of buttonquail are named for their superficial resemblance to quail, and form the family Turnicidae in the order Charadriiformes. The king quail, an Old World quail, often is sold in the pet trade, and within this trade is commonly, though mistakenly, referred to as a "button quail". Many of the common larger species are farm-raised for table food or egg consumption, and are hunted on game farms or in the wild, where they may be released to supplement the wild population, or extend into areas outside their natural range. In 2007, 40 million quail were produced in the U.S. New World *Genus ''Callipepla'' **Scaled quail, (commonly called blue quail) ''Callipepla squamata'' **E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-3D-balls.png)