|
Ammoidin
Methoxsalen, sold under the brand name Oxsoralen among others, is a medication used to treat psoriasis, eczema, vitiligo, and some cutaneous lymphomas in conjunction with exposing the skin to ultraviolet (UVA) light from lamps or sunlight. Methoxsalen modifies the way skin cells receive the UVA radiation, allegedly clearing up the disease. Levels of individual patient PUVA exposure were originally determined using the Fitzpatrick scale. The scale was developed after patients demonstrated symptoms of phototoxicity after oral ingestion of methoxsalen followed by PUVA therapy. Chemically, methoxsalen belongs to a class of organic natural molecules known as furanocoumarins. They consist of coumarin annulated with furan. It can also be injected and used topically. Natural sources In 1947, methoxsalen was isolated (under the name "ammoidin") from the plant ''Ammi majus'', bishop's weed. In 1970, Nielsen extracted 8-methoxypsoralen from four species of the genus ''Heracleum (plant), H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Goods Administration
The Therapeutic Goods Administration (TGA) is the medicine and therapeutic regulatory agency of the Australian Government. As part of the Department of Health and Aged Care, the TGA regulates the quality, supply and advertising of medicines, pathology devices, medical devices, blood products and most other therapeutics. Any items that claim to have a therapeutic effect, are involved in the administration of medication, or are otherwise covered by the ''Therapeutic Goods Act 1989'', the ''Therapeutic Goods Regulations 1990'', or a ministerial order, must be approved by the TGA and registered in the Australian Register of Therapeutic Goods. Structure of the TGA and medical regulation in Australia In Australia, medical products are regulated by the TGA and, for controlled drugs such as cannabis, the Office of Drug Control (ODC). Together, the TGA and ODC form the Health Products Regulation Group within the Department of Health and Aged Care. The Health Products Regulation Group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heracleum (plant)
''Heracleum'' is a genus of biennial and perennial herbs in the carrot family Apiaceae. They are found throughout the temperate northern hemisphere and in high mountains as far south as Ethiopia. Common names for the genus or its species include hogweed and cow parsnip. The genus name ''Heracleum'' was described by Carl Linnaeus in 1753. It derives from the Ancient Greek () "of Heracles", referring to the mythological hero. Species Many species of the genus ''Heracleum'' are similar in appearance. An outlier is ''H. mantegazzianum'', the large size of which is exceptional. Common species include: * ''Heracleum mantegazzianum'', giant hogweed, native to the western Caucasus region of Eurasia, a serious invasive species in many areas of Europe and North America * ''Heracleum sosnowskyi'', Sosnowsky's hogweed, native to the eastern Caucasus, a common weed throughout Europe and Asia * ''Heracleum persicum'', Persian hogweed, native to Iran, Iraq, and Turkey * ''Heracleum spho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamate 4-hydroxylase
In enzymology, a trans-cinnamate 4-monooxygenase () is an enzyme that catalyzes the chemical reaction :trans-cinnamate + NADPH + H+ + O2 \rightleftharpoons 4-hydroxycinnamate + NADP+ + H2O The 4 substrates of this enzyme are trans-cinnamate, NADPH, H+, and O2, whereas its 3 products are 4-hydroxycinnamate, NADP+, and H2O. This enzyme participates in phenylalanine metabolism and phenylpropanoid biosynthesis. It employs one cofactor, heme. This enzyme belongs to the family of oxidoreductases, specifically those acting on paired donors, with O2 as oxidant and incorporation or reduction of oxygen. The oxygen incorporated need not be derived from O2 with NADH or NADPH as one donor, and incorporation of one atom o oxygen into the other donor. Nomenclature The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylation
In chemistry, hydroxylation can refer to: *(i) most commonly, hydroxylation describes a chemical process that introduces a hydroxyl group () into an organic compound. *(ii) the ''degree of hydroxylation'' refers to the number of OH groups in a molecule. The ''pattern of hydroxylation'' refers to the location of hydroxy groups on a molecule or material. Hydroxylation reactions Synthetic hydroxylations Installing hydroxyl groups into organic compounds can be effected by various metal catalysts. Many such catalysts are biomimetic, i.e. they are inspired by or intended to mimic enzymes such as cytochrome P450. Whereas many hydroxylations insert O atoms into bonds, some reactions ''add'' OH groups to unsaturated substrates. The Sharpless dihydroxylation is such a reaction: it converts alkenes into diols. The hydroxy groups are provided by hydrogen peroxide, which adds across the double bond of alkenes. Biological hydroxylation In biochemistry, hydroxylation reactions are often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamic Acid
Cinnamic acid is an organic compound with the formula C6H5-CH=CH- COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a ''cis'' and a ''trans'' isomer, although the latter is more common. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like odor; it and its more volatile ethyl ester (ethyl cinnamate) are flavor components ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the formula . It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin. It is encoded by the codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement for its analgesic and antidepressant effects. It is a direct precursor to the neuromodulator phenethylamine, a commonly used dietary supplement. As an essential amino acid, phenylalanine is not s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropanoid
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and also mediate plant-pollinator interactions as floral pigments and scent compounds. Hydroxycinnamic acids Phenylalanine is first converted to cinnamic aci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Umbelliferone
Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and ''beta''-umbelliferone, is a natural product of the coumarin family. It absorbs ultraviolet light strongly at several wavelengths. There are some indications that this chemical is antimutagenic, it is used in sunscreens. Umbelliferone has been reported to have antioxidant properties. It is a yellowish-white crystalline solid that has a slight solubility in hot water, but high solubility in ethanol. Natural occurrences and name Umbelliferone's name is from the umbelliferae family of plants, and the plant family in turn was named for their umbrella-shaped inflorescences, each called an umbel. Umbelliferone occurs in many familiar plants from the Apiaceae (Umbelliferae) family such as carrot, coriander and garden angelica, as well as in plants from other families, such as the mouse-ear hawkweed (''Hieracium pilosella'', Asteraceae) or the bigleaf hydrangea (''Hydrangea macrophylla'', Hydrangeaceae, under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
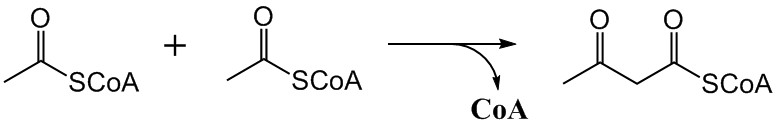
Mevalonate Pathway
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Umbelliferone
Umbelliferone, also known as 7-hydroxycoumarin, hydrangine, skimmetine, and ''beta''-umbelliferone, is a natural product of the coumarin family. It absorbs ultraviolet light strongly at several wavelengths. There are some indications that this chemical is antimutagenic, it is used in sunscreens. Umbelliferone has been reported to have antioxidant properties. It is a yellowish-white crystalline solid that has a slight solubility in hot water, but high solubility in ethanol. Natural occurrences and name Umbelliferone's name is from the umbelliferae family of plants, and the plant family in turn was named for their umbrella-shaped inflorescences, each called an umbel. Umbelliferone occurs in many familiar plants from the Apiaceae (Umbelliferae) family such as carrot, coriander and garden angelica, as well as in plants from other families, such as the mouse-ear hawkweed (''Hieracium pilosella'', Asteraceae) or the bigleaf hydrangea (''Hydrangea macrophylla'', Hydrangeaceae, under ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shikimate
Shikimic acid, more commonly known as its anionic form shikimate, is a cyclohexene, a cyclitol and a cyclohexanecarboxylic acid. It is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower ''shikimi'' (, the Japanese star anise, ''Illicium anisatum''), from which it was first isolated in 1885 by Johan Fredrik Eykman. The elucidation of its structure was made nearly 50 years later. Biosynthesis Phosphoenolpyruvate and erythrose-4-phosphate condense to form 3-deoxy-D-arabinoheptulosonate-7-phosphate (DAHP), in a reaction catalyzed by the enzyme DAHP synthase. DAHP is then transformed to 3-dehydroquinate (DHQ), in a reaction catalyzed by DHQ synthase. Although this reaction requires nicotinamide adenine dinucleotide (NAD) as a cofactor, the enzymic mechanism regenerates it, resulting in the net use of no NAD. : DHQ is dehydrated to 3-dehydroshikimic acid by the enzyme 3-dehydroquinate dehydratase, which is reduced to shi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bergapten
Bergapten (5-methoxypsoralen) is a naturally-occurring organic chemical compound produced by numerous plant species, especially from the carrot family Apiaceae and the citrus family Rutaceae. For example, bergapten has been extracted from 24 species of the genus '' Heracleum'' in the family Apiaceae. Cited by Mitchell and Rook (1979). In the family Rutaceae, various ''Citrus'' species contain significant amounts of bergapten, especially the bergamot orange, the micrantha, and certain varieties of lime and bitter orange. Bergapten belongs to a class of chemical compounds known as the furanocoumarins. In 1834, Kalbrunner isolated 5-methoxypsoralen from bergamot essential oil, hence the common name "bergapten". It was the first furanocoumarin to be isolated and identified. Toxicity Bergapten is a derivative of psoralen, the parent compound of a family of naturally-occurring organic compounds known as the linear furanocoumarins (so called since they exhibit a linear chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_I_(cropped).jpg)