|
Ammeline
Ammeline (4,6-diamino-2-hydroxy-1,3,5-triazine) is a triazine derivative. It is the hydrolysis product of melamine. Synthesis Ammeline can be synthesized by the pyrolysis of urea or the condensation reaction among 2 moles of dicyandiamide and 1 mole of biuret. : 2 C2H4N4 + C2H5N3O2 → 2C3H5N5O + NH3 Chemical properties Ammeline is weakly acidic with pKa ~9. It can form nitrate, sulfate, chromate, and oxalate salts. Ammeline reacts with boiling dilute hydrochloric acid to form melem and ammonia. Ammeline is the first step in melamine hydrolysis. Further hydrolysis (e.g. boiling ammeline with dilute alkali) yields ammelide. References * {{cite journal , author = B. Bann and S.A. Miller , title = Melamines and derivatives of melamine , journal = Chemical Reviews ''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by Willia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melamine
Melamine is an organic compound with the formula C3H6N6. This white solid is a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 67% nitrogen by mass, and its derivatives have fire retardant properties due to its release of nitrogen gas when burned or charred. Melamine can be combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high pressure decorative laminates such as Formica, melamine dinnerware, laminate flooring, and dry erase boards. Melamine foam is used as insulation, soundproofing material and in polymeric cleaning products, such as Magic Eraser. Melamine was once illegally added to baby formula in China, in order to increase the apparent protein content. Ingestion of melamine may lead to reproductive damage, or bladder or kidney stones, and bladder cancer. It is also an irritant when inhaled or in contact with the skin or eyes. The United N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammelide
, Section2={{Chembox Properties , Formula = C3H4N4O2 , MolarMass = 128.09 g/mol , Appearance = white powder , Density = , MeltingPt = , MeltingPt_notes = , BoilingPt = , BoilingPt_notes = , Solubility = insoluble , SolubleOther = soluble in concentrated mineral acids, alkalis and ammonia , Solvent = , pKa = , pKb = , Section7={{Chembox Hazards , MainHazards = , NFPA-H = , NFPA-F = , NFPA-R = , NFPA-S = , FlashPt = , AutoignitionPt = , ExploLimits = , PEL = Ammelide (6-amino-2,4-dihydroxy-1,3,5-triazine) is a triazine and the hydrolysis product of ammeline. Synthesis Ammelide can be obtained by heating dicyandiamide with aqueous ammonia at 160−170 °C. It can also be synthesized by heating melam with concentrated sulfuric acid for a short time at 190 °C. Chemical property Ammelide forms salts with both acids (hydrochloric acid, nitric acid, sulfuric acid) and bases (sodium hydroxide, ammonium, calcium hydroxide). Ammelide decom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl oxalate (C2O4(CH3)2). It is a conjugate base of oxalic acid. At neutral pH in aqueous solution, oxalic acid converts completely to oxalate. Relationship to oxalic acid The dissociation of protons from oxalic acid proceeds in a stepwise manner; as for other polyprotic acids, loss of a single proton results in the monovalent hydrogenoxalate anion . A salt with this anion is sometimes called an acid oxalate, monobasic oxalate, or hydrogen oxalate. The equilibrium constant ( ''K''a) for loss of the first proton is (p''K''a = 1.27). The loss of the second proton, which yields the oxalate ion, has an equilibrium constant of (p''K''a = 4.28). These values imply, in solutions with neutral pH, no oxalic acid and only trace am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reviews
''Chemical Reviews'' is peer-reviewed scientific journal published twice per month by the American Chemical Society. It publishes review articles on all aspects of chemistry. It was established in 1924 by William Albert Noyes (University of Illinois). the editor-in-chief is Sharon Hammes-Schiffer. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, CAB International, EBSCOhost, ProQuest, PubMed, Scopus, and the Science Citation Index. According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 60.622. See also * Accounts of Chemical Research ''Accounts of Chemical Research'' is a semi-monthly peer-reviewed scientific journal published by the American Chemical Society containing overviews of basic research and applications in chemistry and biochemistry. It was established in 1968 and th ... References External links * American Chemical Society academic journals Review journals Monthly journals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melem
In chemistry Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ..., melem is a compound with formula ; specifically, 2,5,8-triamino-heptazine or 2,5,8-triamino-tri-''s''-triazine, whose molecule can be described as that of heptazine with the three hydrogen atoms replaced by amino groups. It is a white crystalline solid. Preparation Melem can be prepared by thermal decomposition of various C−N−H compounds, such as melamine C3N3(NH2)3, dicyandiamide H4C2N4, ammonium dicyanamide NH4 (CN)2 cyanamide H2CN2, at 400 to 450 °C. Structure and properties Crystal structure Melem crystallizes in the group P21/c (No. 14), with parameters a = 739.92(1) pm, b = 865.28(3) pm, c = 1338.16(4) pm, β = 99.912(2)°, and Z = 4. The almost-planar molecules are arranged in parallel layers s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
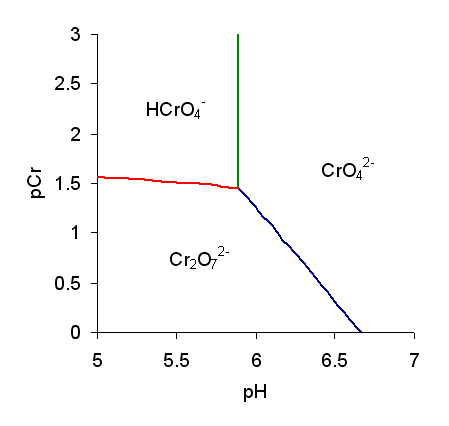
Monochromate
Chromate salts contain the chromate anion, . Dichromate salts contain the dichromate anion, . They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible. Chemical properties Potassium-chromate-sample.jpg, potassium chromate Potassium-dichromate-sample.jpg, potassium dichromate Chromates react with hydrogen peroxide, giving products in which peroxide, , replaces one or more oxygen atoms. In acid solution the unstable blue peroxo complex Chromium(VI) oxide peroxide, CrO(O2)2, is formed; it is an uncharged covalent molecule, which may be extracted into ether. Addition of pyridine results in the formation of the more stable complex CrO(O2)2py. Acid–base properties In aqueous solution, chromate and dichromate anions exist in a chemical equilibrium. :2 + 2 H+ + H2O The predominance diagram shows that the position of the equilibrium depends on b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-3D-balls.png)