|
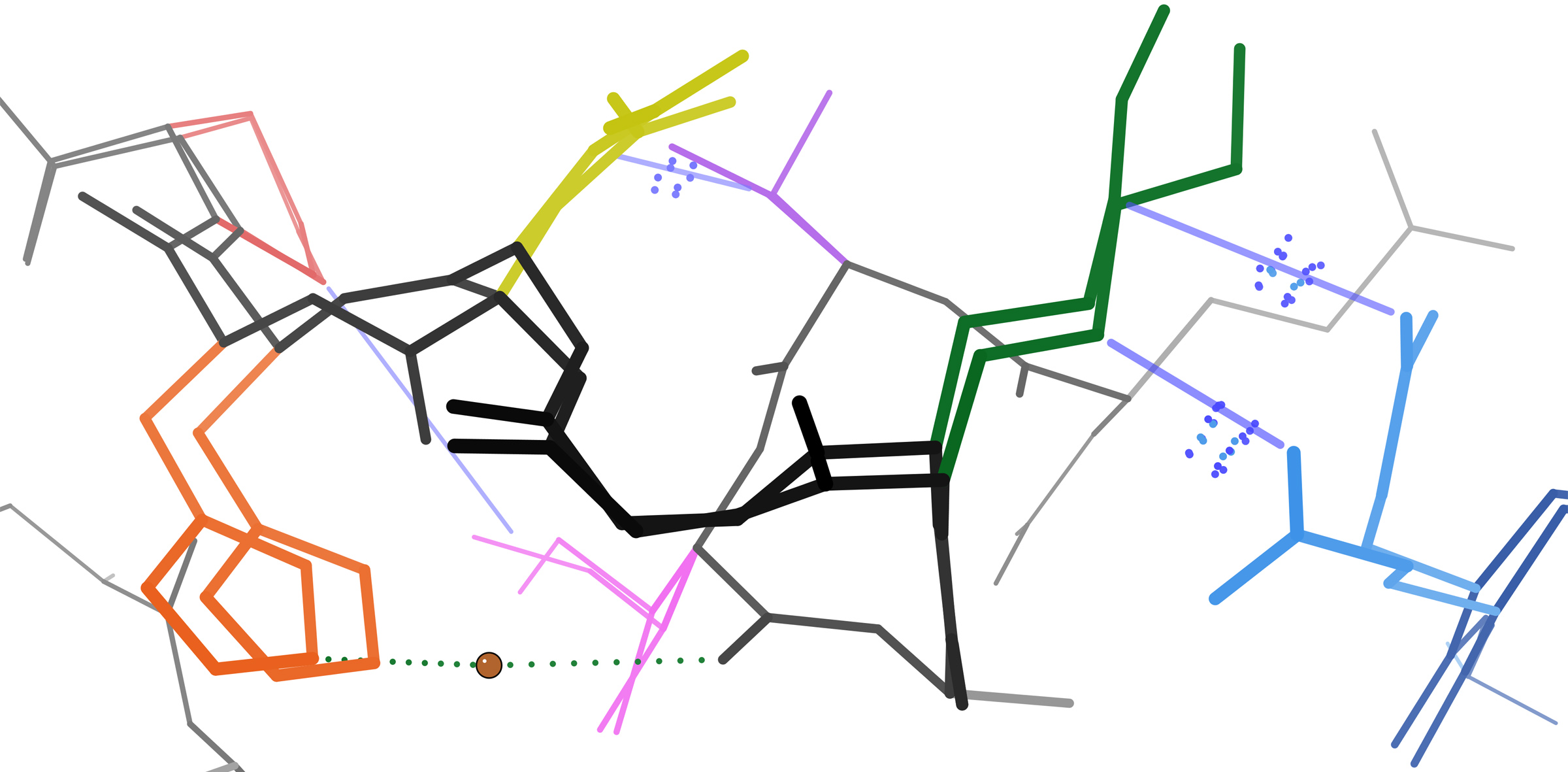
Adherens Junctions
Adherens junctions (or zonula adherens, intermediate junction, or "belt desmosome") are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell (zonula adherens) or as spots of attachment to the extracellular matrix (focal adhesion). Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells. A similar cell junction in non-epithelial, non-endothelial cells is the fascia adherens. It is structurally the same, but appears in ribbonlike patterns that do not completely encircle the cells. One example is in cardiomyocytes. Proteins Adherens junctions are composed of the following proteins: * cadherins. The cadherins are a family of transmembrane proteins tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tight Junctions
Tight junctions, also known as occluding junctions or ''zonulae occludentes'' (singular, ''zonula occludens''), are multiprotein junctional complexes whose canonical function is to prevent leakage of solutes and water and seals between the epithelial cells. They also play a critical role maintaining the structure and permeability of endothelial cells. Tight junctions may also serve as leaky pathways by forming selective channels for small cations, anions, or water. The corresponding junctions that occur in invertebrates are septate junctions. Structure Tight junctions are composed of a branching network of sealing strands, each strand acting independently from the others. Therefore, the efficiency of the junction in preventing ion passage increases exponentially with the number of strands. Each strand is formed from a row of transmembrane proteins embedded in both plasma membranes, with extracellular domains joining one another directly. There are at least 40 different protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the mobility and contraction of cells during cell division. Actin participates in many important cellular processes, including muscle contraction, cell motility, cell division and cytokinesis, vesicle and organelle movement, cell sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fascia Adherens
In anatomy for cardiac muscle, fascia adherens are ribbon-like structures that stabilize non-epithelial tissue. They are similar in function and structure to the zonula adherens or adherens junction of epithelial cells. It is a broad intercellular junction in the transversal sections of an intercalated disc of cardiac muscle anchoring actin filaments. It helps to transmit contractile forces.Histology" A Text and Atlas with Correlated Cell and Molecular Biology by Michael H. Ross and Mojciech Pawlina 5th edition See also *Fascia A fascia (; plural fasciae or fascias; adjective fascial; from Latin: "band") is a band or sheet of connective tissue, primarily collagen, beneath the skin that attaches to, stabilizes, encloses, and separates muscles and other internal organs. ... References Cardiovascular system Fascia {{muscle-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
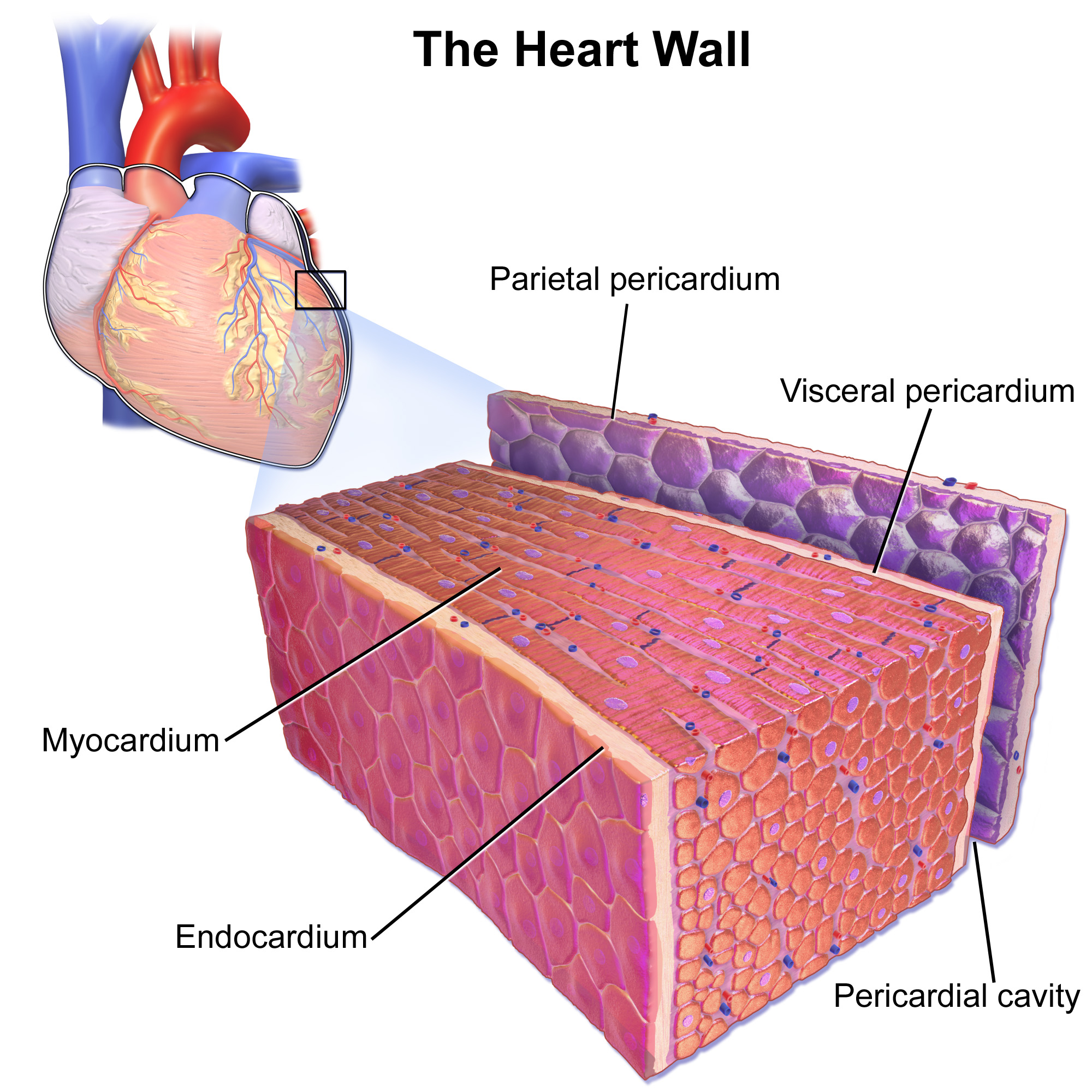
Cardiomyocytes
Cardiac muscle (also called heart muscle, myocardium, cardiomyocytes and cardiac myocytes) is one of three types of vertebrate muscle tissues, with the other two being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that constitutes the main tissue of the wall of the heart. The cardiac muscle (myocardium) forms a thick middle layer between the outer layer of the heart wall (the pericardium) and the inner layer (the endocardium), with blood supplied via the coronary circulation. It is composed of individual cardiac muscle cells joined by intercalated discs, and encased by collagen fibers and other substances that form the extracellular matrix. Cardiac muscle contracts in a similar manner to skeletal muscle, although with some important differences. Electrical stimulation in the form of a cardiac action potential triggers the release of calcium from the cell's internal calcium store, the sarcoplasmic reticulum. The rise in calcium causes the cell's my ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadherins
Cadherins (named for "calcium-dependent adhesion") are a type of cell adhesion molecule (CAM) that is important in the formation of adherens junctions to allow cells to adhere to each other . Cadherins are a class of type-1 transmembrane proteins, and they are dependent on calcium (Ca2+) ions to function, hence their name. Cell-cell adhesion is mediated by extracellular cadherin domains, whereas the Cadherin cytoplasmic region, intracellular cytoplasmic tail associates with numerous adaptors and signaling proteins, collectively referred to as the cadherin adhesome. The cadherin family is essential in maintaining the cell-cell contact and regulating cytoskeletal complexes. The cadherin superfamily includes cadherins, protocadherins, desmogleins, desmocollins, and more. In structure, they share ''cadherin repeats'', which are the extracellular Ca2+-binding domains. There are multiple classes of cadherin molecules, each designated with a prefix (in general, noting the types of tissue w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Delta Catenin
Delta-1-catenin and Delta-2-catenin are members of a subfamily of proteins with ten Armadillo-repeats. Delta-2-catenin is expressed in the brain where it is important for normal cognitive development. Like beta-catenin and gamma-catenin, delta-catenins seem to interact with Presenilins. These catenin-presenilin interaction have implications for cadherin function and regulation of cell-to-cell adhesion. While beta-catenin acts as a transcription regulatory protein in the Wnt/TCF pathway, delta-catenin 1 has been implicated as a regulator of the NF-κB transcription factor. Palmitoylation of delta-catenin seems to coordinate activity-dependent changes in synaptic adhesion molecules, synapse structure, and receptor localizations that are involved in memory formation. References See also *Catenin *CTNND1 p120, and called catenin delta-1 is a protein that in humans is encoded by the CTNND1 gene. Function This gene encodes a member of the Armadillo protein family, which fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
γ-catenin
Plakoglobin, also known as junction plakoglobin or gamma-catenin, is a protein that in humans is encoded by the ''JUP'' gene. Plakoglobin is a member of the catenin protein family and homologous to β-catenin. Plakoglobin is a cytoplasmic component of desmosomes and adherens junctions structures located within intercalated discs of cardiac muscle that function to anchor sarcomeres and join adjacent cells in cardiac muscle. Mutations in plakoglobin are associated with arrhythmogenic right ventricular dysplasia. Structure Human plakoglobin is 81.7 kDa in molecular weight and 745 amino acids long. The ''JUP'' gene contains 13 exons spanning 17 kb on chromosome 17q21. Plakoglobin is a member of the catenin family, since it contains a distinct repeating amino acid motif called the armadillo repeat. Plakoglobin is highly similar to β-catenin; both have 12 armadillo repeats as well as N-terminal and C-terminal globular domains of unknown structure. Plakoglobin was originally identified ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plakoglobin
Plakoglobin, also known as junction plakoglobin or gamma-catenin, is a protein that in humans is encoded by the ''JUP'' gene. Plakoglobin is a member of the catenin protein family and homologous to β-catenin. Plakoglobin is a cytoplasmic component of desmosomes and adherens junctions structures located within intercalated discs of cardiac muscle that function to anchor sarcomeres and join adjacent cells in cardiac muscle. Mutations in plakoglobin are associated with arrhythmogenic right ventricular dysplasia. Structure Human plakoglobin is 81.7 kDa in molecular weight and 745 amino acids long. The ''JUP'' gene contains 13 exons spanning 17 kb on chromosome 17q21. Plakoglobin is a member of the catenin family, since it contains a distinct repeating amino acid motif called the armadillo repeat. Plakoglobin is highly similar to β-catenin; both have 12 armadillo repeats as well as N-terminal and C-terminal globular domains of unknown structure. Plakoglobin was originally identified ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-catenin
Alpha-catenin functions as the primary protein link between cadherins and the actin cytoskeleton. It has been reported that the actin binding proteins vinculin and alpha-actinin can bind to alpha-catenin. It has been suggested that alpha-catenin does not bind with high affinity to both actin filaments and the E-cadherin-beta-catenin complex at the same time. It has been observed that when alpha-catenin is not in a molecular complex with beta-catenin, it dimerizes and functions to regulate actin filament assembly, possibly by competing with Arp2/3 protein. Alpha catenin exhibits significant protein dynamics. However, a protein complex including a cadherin, actin, beta-catenin and alpha-catenin has not been isolated. The amino acid sequence of alpha-catenin has sequence similarity to that of vinculin. Types Three alpha-catenin genes are expressed in humans: * CTNNA1, alpha-1-catenin (also called alpha-E-catenin) * CTNNA2, alpha-2-catenin (also called alpha-N-catenin) * CTNNA3, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-catenin
Catenin beta-1, also known as beta-catenin (β-catenin), is a protein that in humans is encoded by the ''CTNNB1'' gene. Beta-catenin is a dual function protein, involved in regulation and coordination of cell–cell adhesion and gene transcription. In humans, the CTNNB1 protein is encoded by the ''CTNNB1'' gene. In ''Drosophila'', the homologous protein is called ''armadillo''. β-catenin is a subunit of the cadherin protein complex and acts as an intracellular signal transducer in the Wnt signaling pathway. It is a member of the catenin protein family and homologous to γ-catenin, also known as plakoglobin. Beta-catenin is widely expressed in many tissues. In cardiac muscle, beta-catenin localizes to adherens junctions in intercalated disc structures, which are critical for electrical and mechanical coupling between adjacent cardiomyocytes. Mutations and overexpression of β-catenin are associated with many cancers, including hepatocellular carcinoma, colorectal carcinoma, lung c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dynamics
Proteins are generally thought to adopt unique structures determined by their amino acid sequences. However, proteins are not strictly static objects, but rather populate ensembles of (sometimes similar) conformations. Transitions between these states occur on a variety of length scales (tenths of Å to nm) and time scales (ns to s), and have been linked to functionally relevant phenomena such as allosteric signaling and enzyme catalysis. The study of protein dynamics is most directly concerned with the transitions between these states, but can also involve the nature and equilibrium populations of the states themselves. These two perspectives—kinetics and thermodynamics, respectively—can be conceptually synthesized in an "energy landscape" paradigm: highly populated states and the kinetics of transitions between them can be described by the depths of energy wells and the heights of energy barriers, respectively. Local flexibility: atoms and residues Portions of protein s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |